Ζ. Kristallogr. NCS 217 (2002) 4 8 5 ^ 8 6
© by Oldenbourg Wissenschaftsverlag, München
485
Crystal structure of the low-temperature modification of trilithium heptaarsenide, LT-LÌ3AS7
W. Hönle*·
1, J. Buresch", K. Peters", J. H. Chang" and H. G. v o n Schnering"
' Max-Planck-Institut für Chemische Physik fester Stoffe, Nöthnitzer Str. 40, D-01187 Dresden, Germany
" Max-Planck-Institut für Festkörperforschung. Heisenbergstr. 1, D-70569 Stuttgart, Germany Received September 19, 2002, accepted and available on-line November 5, 2002; CSD-No. 409657
AS
7(1)
Abstract
AS7L13, orthorhombic, Pbca (No. 61), a = 12.466(3) Â, b = 22.489(4) Â, c = 12.592(2) Ä, V= 3530.1 À3
, Z = 16, Rgi(F) = 0.055, wR
rei(F2) = 0.132, 7 = 293 K.Source of material
L13AS7 is formed by reaction of stoichiometric mixtures of the ele- ments in welded Nb-tubes (12 h heating to 903 K; 48 h reaction time at 903 K; cooling to RT within 36 h). The light grey colour is in accordance with the band gap 2.0 eV (from diffuse reflectivity spectra, powder). LT-LÌ3AS7 transforms at 728(10) Κ to HT-L13AS7 [1,2]. The compound is very sensitive to oxidation and hydrolysis.
Discussion
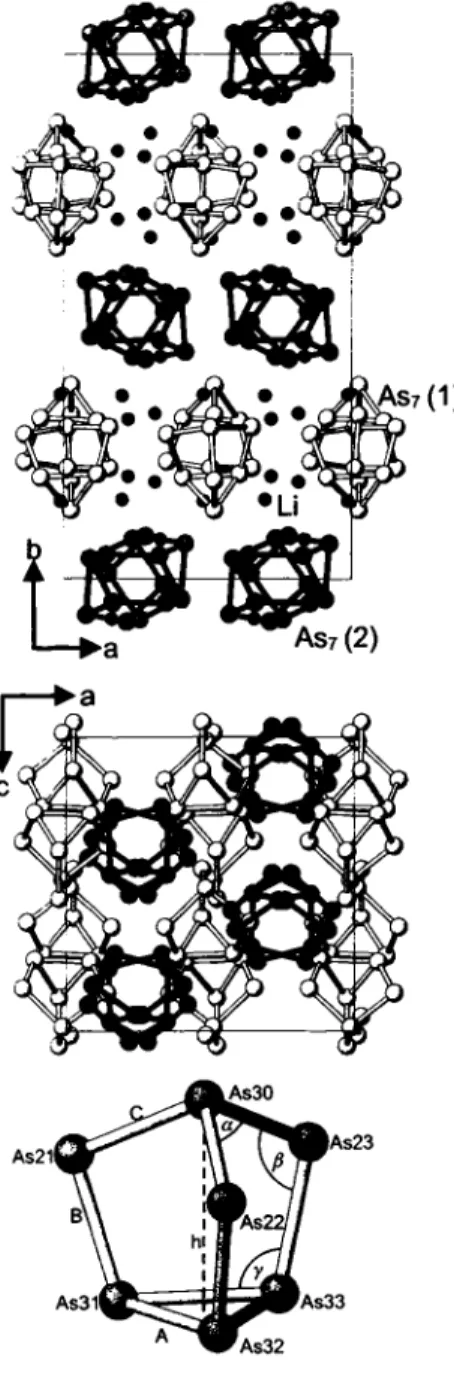
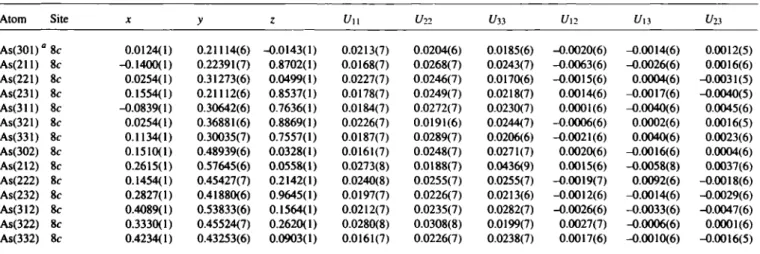
LT-L13AS7 forms a slightly changed o f 160 structure of the LT-Rb3As7 type [3]. The arrangement of the two crystallographi- cally independent Zintl anions (As?)*~ represents a hierarchical clus- ter-replacement structure [4] of the hexagonal a-La type (however, more distorted than that in LT-Rb3As7). The structure of HT-LÌ3AS7 is not yet known [1,2]. The mean As—As bond lengths and the bond angles (A = 2.495 Χ, Β = 2.401 Â, C = 2.424 Â, a = 100.5°, β = 101.5°, γ = 104,5°, Q = h/A = 3.446 / 2.495 = 1.38 (see lower figure) show that the cluster anions belong to the "less ionic"
nortricyclenes [5,6], The Li
+cations connect 3 - 4 (AS7)
3-anions (d(Li—As) = 2.66 Â - 3.13 Â) indicating the preformation of LÌ3AS7 molecules [6,7] which are ideal precursors for the solvatisation of Zintl compounds [8],
Table 1. Data collection and handling.
Crystal:
Wavelength:
μ·
Diffractometer, scan mode:
2θπωχ:
N(hkl)measured, N(AW)umquc:
Criterion for /obs, N(hkl)gc.
N(param)nfmai:
Programs:
light grey irregular polyhedron, size 0 . 1 5 x 0 . 1 5 x 0 . 2 mm Mo Ka radiation (0.71073 À) 260.83 c n f1
Siemens PI, ω 55°
3418,3418
/obs > 2 ff(7otJ, 2641
151SHELXS-97 [9], SHELXL-97 [10]
ATOMS [11]
Table 2. Atomic coordinates and displacement parameters (in Â2).
Atom Site X y ζ
t /íso
Li(l) 8c 0.382(2) 0.519(1) 0.917(2) 0.030(6) Li(2) 8c 0.487(3) 0.352(1) 0.248(3) 0.042(7) Li(3) 8c -0.163(3) 0.424(1) 0.755(3) 0.043(7) Li(4) 8c 0.223(3) 0.305(1) 0.955(2) 0.040(7) Li(5) 8c 0.184(3) 0.685(1) 0.994(3) 0.044(7) Li(6) 8c 0.201(3) 0.349(2) 0.166(3) 0.055(9)
* Correspondence author (e-mail: hoenle@cpfs.mpg.de)
486
LT-LÌ3ASTable 3. Atomic coordinates and displacement parameters (in Â2).
Atom Site X y ζ υ η t/22 t/33 t/12 U ,3 t/23
As(301 " 8c 0.0124(1) 0.21114(6) -0.0143(1) 0.0213(7) 0.0204(6) 0.0185(6) -0.0020(6) -0.0014(6) 0.0012(5) As(211 8c -0.1400(1) 0.22391(7) 0.8702(1) 0.0168(7) 0.0268(7) 0.0243(7) -0.0063(6) -0.0026(6) 0.0016(6) As(221 8c 0.0254(1) 0.31273(6) 0.0499(1) 0.0227(7) 0.0246(7) 0.0170(6) -0.0015(6) 0.0004(6) -0.0031(5) As(231 8c 0.1554(1) 0.21112(6) 0.8537(1) 0.0178(7) 0.0249(7) 0.0218(7) 0.0014(6) -0.0017(6) -0.0040(5) As(311 8c -0.0839(1) 0.30642(6) 0.7636(1) 0.0184(7) 0.0272(7) 0.0230(7) 0.0001(6) -0.0040(6) 0.0045(6) As(321 8c 0.0254(1) 0.36881(6) 0.8869(1) 0.0226(7) 0.0191(6) 0.0244(7) -0.0006(6) 0.0002(6) 0.0016(5) As(331 8c 0.1134(1) 0.30035(7) 0.7557(1) 0.0187(7) 0.0289(7) 0.0206(6) -0.0021(6) 0.0040(6) 0.0023(6) As(302 8c 0.1510(1) 0.48939(6) 0.0328(1) 0.0161(7) 0.0248(7) 0.0271(7) 0.0020(6) -0.0016(6) 0.0004(6) As(212 8c 0.2615(1) 0.57645(6) 0.0558(1) 0.0273(8) 0.0188(7) 0.0436(9) 0.0015(6) -0.0058(8) 0.0037(6) As(222 8c 0.1454(1) 0.45427(7) 0.2142(1) 0.0240(8) 0.0255(7) 0.0255(7) -0.0019(7) 0.0092(6) -0.0018(6) As(232 8c 0.2827(1) 0.41880(6) 0.9645(1) 0.0197(7) 0.0226(7) 0.0213(6) -0.0012(6) -0.0014(6) -0.0029(6) As(312 8c 0.4089(1) 0.53833(6) 0.1564(1) 0.0212(7) 0.0235(7) 0.0282(7) -0.0026(6) -0.0033(6) -0.0047(6) As(322 8c 0.3330(1) 0.45524(7) 0.2620(1) 0.0280(8) 0.0308(8) 0.0199(7) 0.0027(7) -0.0006(6) 0.0001(6) As(332 8c 0.4234(1) 0.43253(6) 0.0903(1) 0.0161(7) 0.0226(7) 0.0238(7) 0.0017(6) -0.0010(6) -0.0016(5)
a: labelling of the As atoms As(ijk) with ι = homoatomic connectivity, y = position in cluster (lower figure), k = number of As7 cluster (upper figure).
References
1. Hönle, W.; Balcarek, J.; Meyer, T. M.; von Schnering, H. G.: Tri- alkalimetallheptaarsenide M3AS7 (M = Li, Na, K) sowie Ké(SbAs6)(As7), Ζ. Kristallogr. Suppl. 9 (1995) 180.
2. Buresch, J.: Binäre und temare Arsenide, Antimonide und Arsenid- Antimonide der Alkalimetalle mit den Anionen Χ3", X35-, XV5-, ¿[Χ-1, X73" und X75". Dissertation, Universität Stuttgart, 1996.
3. Hönle, W.; Buresch, J.; Wolf, J.; Peters, K.; Chang, J.-H.; von Schnering, H. G.: Crystal structure of the low-temperature modification of tri- nibidium heptaarsenide, LT-Rb3As7. Z. Kristallogr. NCS 217 (2002) 489-490.
4. Carrillo-Cabrera, W.; Caroca-Canales, Ν.; von Schnering, H. G.:
K2i-dNa2+¿In39 (<5 = 2.8): A cluster-replacement Clathrate-II-Structure with an Alkali Metal Mi36-Network. Z. Anorg. Allg. Chem. 620 (1994) 247-257.
5. Hönle, W.; von Schnering, H. G.: Die Strukturen der Hepta-Hetero- Nortricyclene P7(Sime3)3 und P4(Sime2)4. Ζ. Anorg. Allg. Chem. 440 (1978) 171-182.
6. von Schnering, H. G.; Hönle, W.: Bridging Chasms with Polyphosphides.
Chem. Rev. 88 (1988) 243-273.
7. Hönle, W.; von Schnering, H. G., Somer, M.: Strukturen von Hepta- arseniden der Alkalimetalle und deren Solvaten. Ζ. Kristallogr. 174 (1986) 82-83.
8. von Schnering, H. G.: Zintl-Phasen: Prinzipien von Struktur und Bindung.
Nova Acta Leopoldina Halle (Saale) 59, Nr. 264 (1985) 165-182.
9. Sheldrick, G. M.: Phase Annealing in SHELX-90: Direct Methods for Larger Structures. Acta Crystallogr. A46 (1990) 467-473.
10. Sheldrick, G. M.: SHELXL-97. Program for the Refinement of Crystal Structures. University of Göttingen, Germany 1997.
11.Dowty, E: ATOMS 5.1. A Complete Program for Displaying Atomic Structures. By Shape software, 521 Hidden Valley Road, Kingsport, TN 37663, USA 2000.