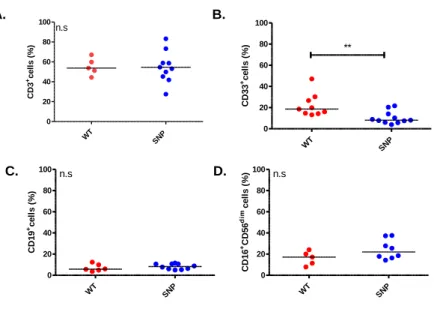
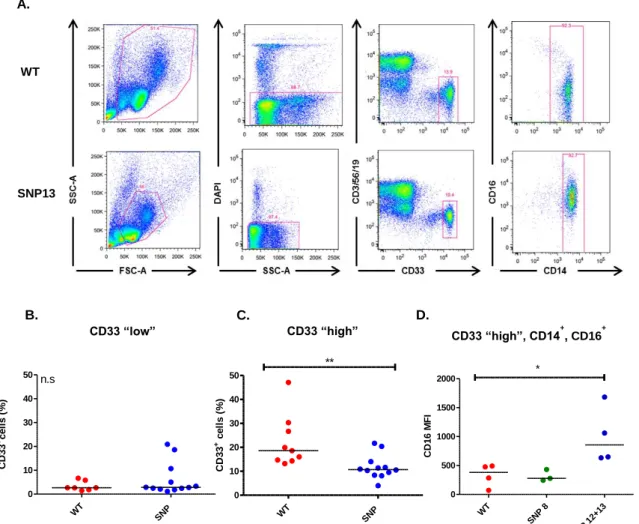
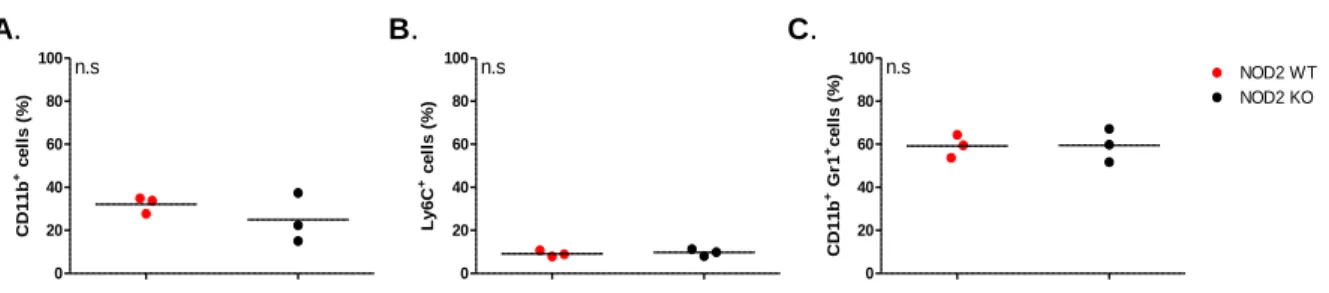
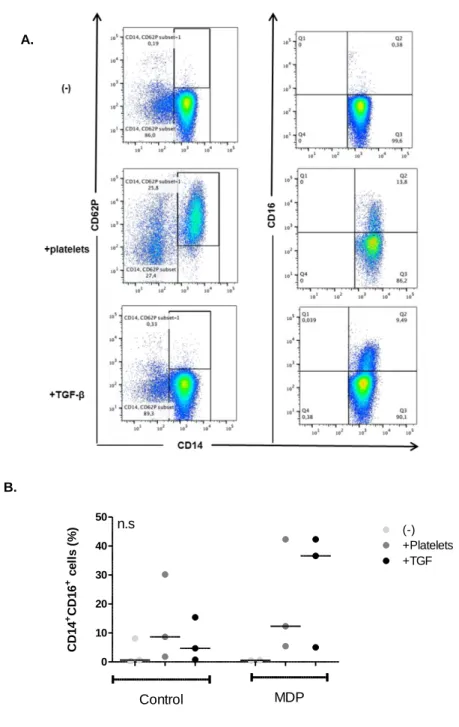
Immunomodulation by NOD2/CARD15 and Vitamin D
Volltext
Abbildung
![Figure 1.1. The three phases of aGvHD, according to Ferrara et al [6]. In phase I, chemotherapy or radiotherapy as part of transplant conditioning causes host tissue damage and release of inflammatory cytokines such as TNF, IL-1β, an](https://thumb-eu.123doks.com/thumbv2/1library_info/3941707.1533414/16.892.262.662.224.583/according-ferrara-chemotherapy-radiotherapy-transplant-conditioning-inflammatory-cytokines.webp)
![Figure 1.3. Development of nTregs and iTregs. Adapted from [66]. nTregs (top) differentiate from naïve conventional T cells to Foxp3+ Tregs in the thymus](https://thumb-eu.123doks.com/thumbv2/1library_info/3941707.1533414/25.892.252.664.586.930/figure-development-ntregs-itregs-adapted-ntregs-differentiate-conventional.webp)
![Figure 1.5. Effects of 1,25(OH) 2 D 3 on various immune cells. Adapted from [80].](https://thumb-eu.123doks.com/thumbv2/1library_info/3941707.1533414/31.892.174.749.191.622/figure-effects-oh-d-various-immune-cells-adapted.webp)
ÄHNLICHE DOKUMENTE
Dies sollte bei der Vit D Dosisanpassung im Falle einer Unterversorgung/ Mangel berücksichtig werden, damit es zu keiner unbeabsichtigten Überdosierung kommt.. Klinisch bewährt
Vitamin D spielt eine zentrale Rolle für die Knochengesund- heit und damit für die Osteoporose-Prophylaxe.. Auch das Immunsystem profitiert von ausreichend Vitamin D, denn es
This may lead to osteoporosis and rickets, and calcium is also involved in nerve function, mu- scle contraction and relaxation, digestion, blood clotting and blood
Laif ® 900 Balance darf nicht eingenommen werden bei bekannter Allergie gegenüber Johanniskraut oder einem der sonstigen Bestandteile?. Hinweise: Ausreichende Erfahrungen über
Eine aktuelle Untersuchung der Universität von Tel Aviv zeigt, dass bei Asthmapatienten mit einem Vi- tamin-D-Dezifit das Risiko, einen Asthmaanfall zu er- leiden, um 25 Prozent
Für die 75 nmol/l sprechen, dass erst ab diesem Zielwert eine Fraktur- und Sturzreduktion er- wartet werden kann (4, 5).. Diesen Ziel- spiegel zu erreichen, empfiehlt denn auch
Da unge - nügende Vitamin-D-Speicher aber für breite Bevölkerungsschichten ein Pro- blem seien, müsse hier die Botschaft lauten: «Geht regelmässig hinaus an die Sonne, holt euch
Zudem zeigt eine weitere Metaanalyse, die sich zurzeit im Druck befindet, dass Kalzium alleine einen neu- tralen Effekt auf nicht vertebrale Frakturen hat (40), ein Resul- tat,