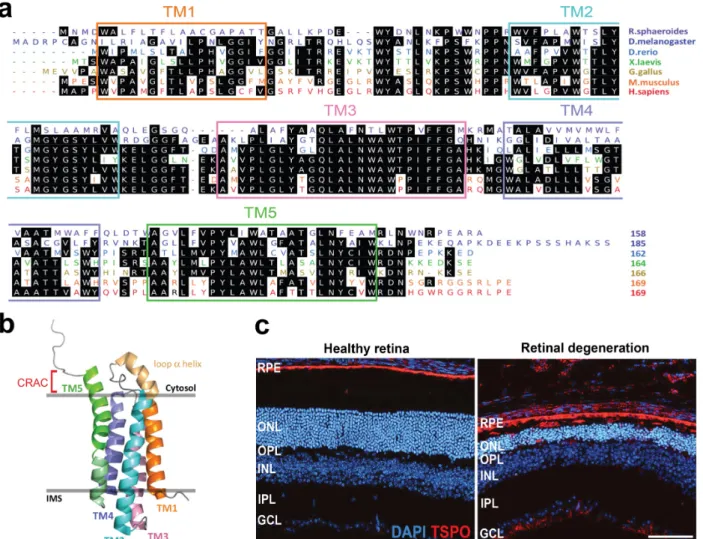
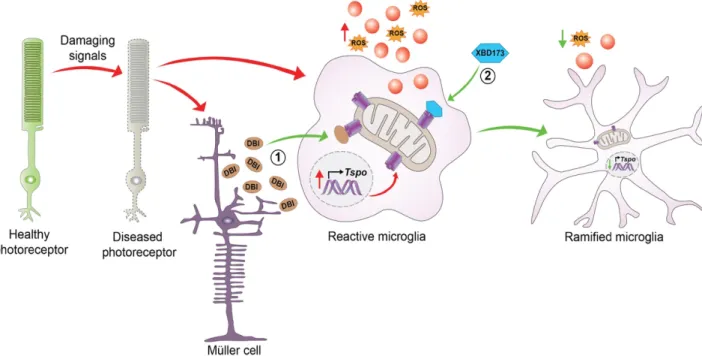
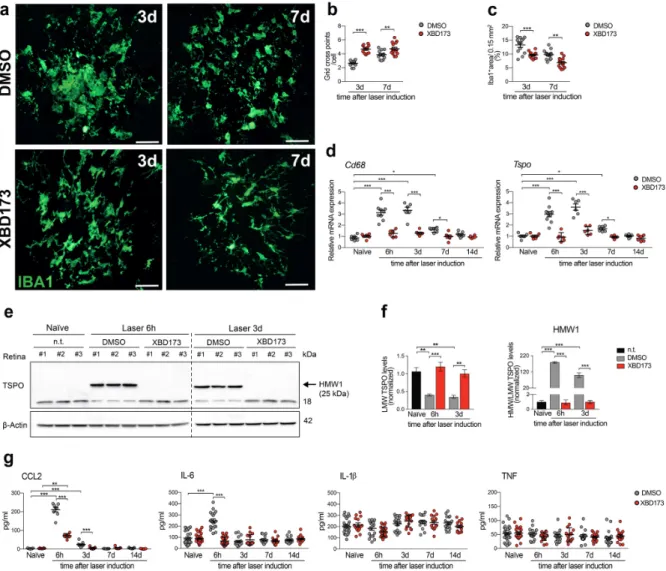
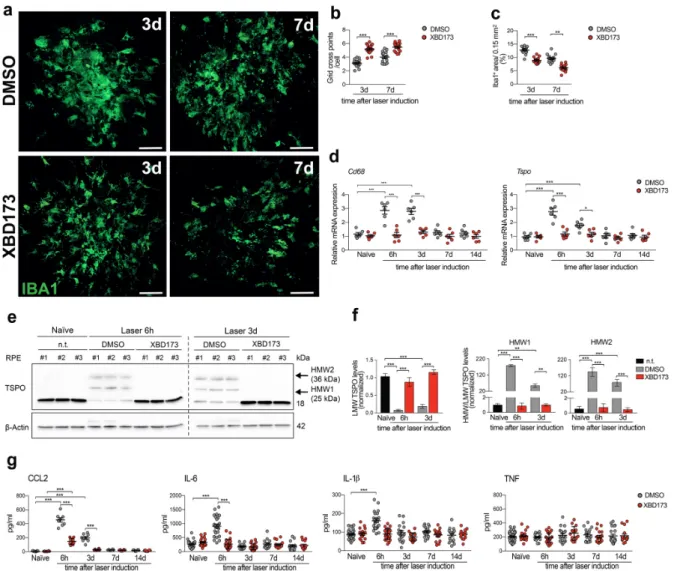
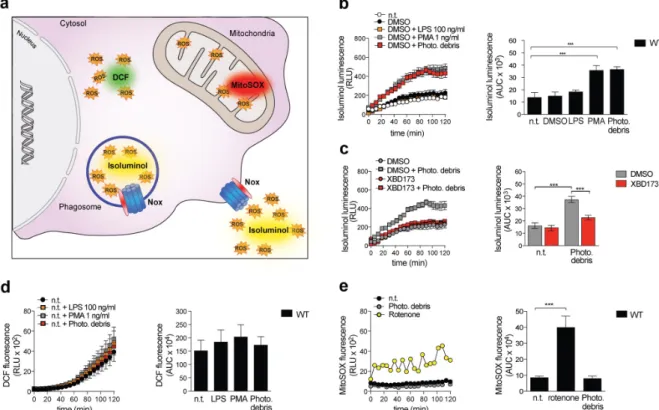
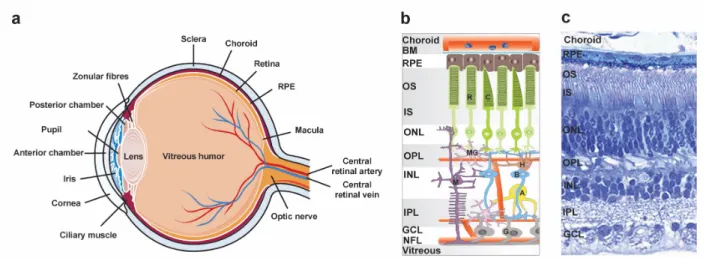
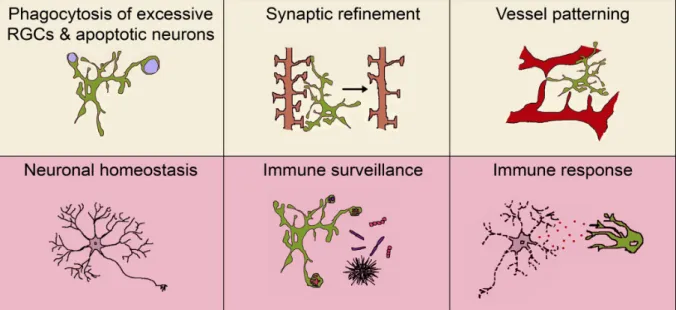
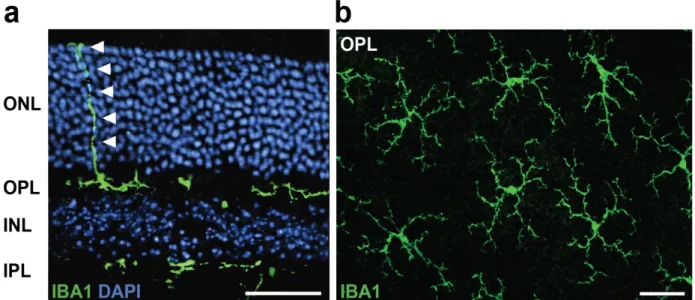
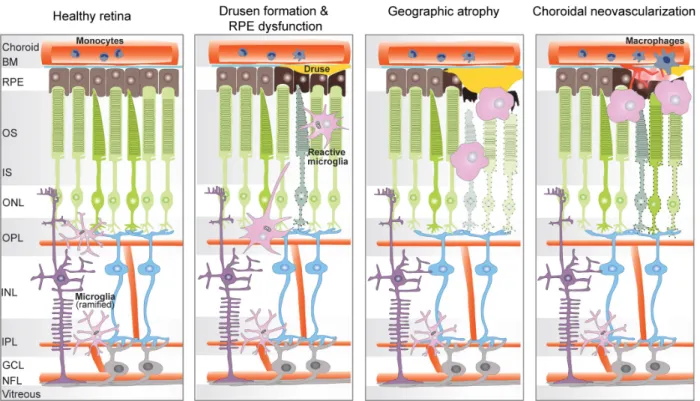
Role of translocator protein (18 kDa) (TSPO) in retinal phagocytes in a mouse model of age-related macular degeneration (AMD)
Volltext
Abbildung
ÄHNLICHE DOKUMENTE
Our contribution is modeling the spatial neighborhood information of a protein directly into a graphical model based on the approach of conditional random fields. CRF is a
To determine whether the expression of Pelo in skin is correlated with the epidermal barrier formation, protein blot analysis of epidermal protein lysates from control
Despite the incremental use of 18 kDa translocator protein positron emission tomography (TSPO-PET) imaging in Alzheimer ’ s disease 9 and β -amyloid mouse models, 10,11 only 2
Amyotrophic lateral sclerosis (ALS) is the most frequent adult onset degenerative disease of the motor neuron (motor neuron disease) with an incidence of
Further support for the common variants in comple- ment factor H (Y402H) and LOC387715 (A69S) genes as ma- jor risk factors for the exudative age-related macular degenera- tion..
In this study, we investigated the potential neuroprotective effects of Emapunil (also known as AC-5216 or XBD-173), a TSPO ligand, in female mice with subacute
However, Stx8 showed no reduction in TRC40-downregulated cells whereas Stx8 was found to be decreased to 62% at steady-state level upon combined WRB/TRC40 knockdown compared to
Amino acid sequences deduced from nucleotide sequences are considered to represent protein tyrosine-specific kinase if they reveal a series of short sequence motifs that are