Original Paper
This article is licensed under the Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 Interna- tional License (CC BY-NC-ND) (http://www.karger.com/Services/OpenAccessLicense). Usage and distribution for commercial purposes as well as any distribution of modified material requires written permission.
© 2015 The Author(s) Published by S. Karger AG, Basel
Department of Physiology, University of Tübingen Gmelinstr. 5, 72076 Tübingen, (Germany)
Tel. +49 7071 29-72194, Fax +49 7071 29-5618, E-Mail florian.lang@uni-tuebingen.de Florian Lang
Involvement of Ca
2+Activated Cl
-Channel Ano6 in Platelet Activation and Apoptosis
Guoxing Liua Guilai Liua Hong Chena Oliver Borsta,b Meinrad Gawazb Andrea Vortkampc Rainer Schreiberd Karl Kunzelmannd Florian Langa
Departments of aPhysiology and bCardiology & Cardiovascular Medicine, Eberhard-Karls-University, Tübingen, cCentre of Medical Biotechnology, University of Essen, Essen, dDepartment of Physiology, University of Regensburg, Regensburg, Germany
Key Words
Cytosolic Ca2+ concentration • Phosphatidylserine translocation • P-selectin • Integrin • Cell volume
Abstract
Background/Aims: The ubiquitously expressed Ca2+ Activated Cl- Channel Ano6 participates in the stimulation of cell membrane scrambling. Defective Ano6 underlies the Scott syndrome, an inherited bleeding disorder with impaired scrambling of plasma membrane phospholipids. At least in theory, the bleeding disorder of Scott syndrome may result from impaired platelet function.
Activators of platelets include thrombin and collagen related peptide (CRP), which trigger increase of cytosolic Ca2+-activity ([Ca2+]i), production of reactive oxygen species (ROS), degranulation, integrin activation, as well as cell shrinkage and phospholipid scrambling of the cell membrane.
The present study thus explored whether Ano6 modifies activation-induced alterations of cytosolic Ca2+-activity ([Ca2+]i), degranulation (P-selectin exposure), integrin activation, phosphatidylserine exposure on the platelet surface and platelet volume. Methods: Platelets from mice lacking Ano6 (ano6-/-) were compared to platelets from corresponding wild-type mice (ano6+/+).
[Ca2+]i was estimated from Fluo-3 fluorescence, ROS from DCFDA fluorescence, degranulation from P-selectin abundance, integrin activation from αIIbβ3-integrin abundance, phosphatidylserine abundance from annexin-V-binding, and cell volume from forward scatter. Results: Platelet number in blood was slightly higher in ano6-/- mice than in ano6+/+ mice. Without activation [Ca2+]i and volume were similar in ano6-/- and ano6+/+ platelets as well as ROS abundance, P-selectin abundance, αIIbβ3 integrin activation, and phosphatidylserine exposure were negligible in both genotypes. Thrombin (0.01 U/ml) and CRP (2 or 5 µg/ml) increased [Ca2+]i, ROS abundance, platelet degranulation, αIIbβ3 integrin activation, and triggered annexin-V-binding as well as cell shrinkage, all effects less pronounced in ano6-/- than in ano6+/+ platelets. Conclusions: Genetic knockout of Ano6 blunts thrombin- and CRP-induced activation and apoptosis of blood platelets.
G. Liu and G. Liu contributed equally and thus share first authorship; K. Kunzelmann and F. Lang contributed equally and thus share last authorship.
Introduction
The ubiquitously expressed cell membrane protein Anoctamin 6 (Ano6; TMEM16F gene) may function as outwardly rectifying Ca2+-dependent and volume-regulated Cl- channel and/or as Ca2+-regulated nonselective Ca2+ permeable cation channel [1-5]. Moreover, Ano6 appears to be essential for Ca2+-mediated scrambling of membrane phospholipids and participates in the regulation of cell blebbing and microparticle shedding [1]. Defective Ano6 leads to Scott syndrome, an inherited bleeding disorder with impaired scrambling of plasma membrane phospholipids [1].
Bleeding disorders may result from impaired function of platelets, cells required for primary haemostasis following vascular injury and by the same token instrumental in thrombosis and vascular occlusion [6, 7]. Moreover, platelets contribute to the pathophysiology of vascular inflammation and atherogenesis [6, 8]. Platelets are activated by increase of cytosolic Ca2+
concentration ([Ca2+]i) [9], which may be accomplished by Ca2+ release from intracellular stores [10] and subsequent activation of the Ca2+ release-activated channel (CRAC) Orai1 (CRACM1) in the plasma membrane [9, 11-13] by the Ca2+ sensing stromal interaction molecule 1 (STIM1) [14]. Orai1/STIM1 thus accomplish store-operated calcium entry (SOCE) [15, 16]. Besides platelet activation [9, 10, 12, 17], Ca2+ triggers cytoskeletal reorganization [18], cell shrinkage and cell membrane scrambling with translocation of phosphatidylserine to the platelet surface [13]. Agonists stimulating platelets include thrombin and collagen related peptide [19, 20]. The present study explored whether Ano6 participates in the regulation of platelet [Ca2+]i, activation and apoptosis. To this end, the impact of Ano6 deletion on Ca2+ entry, platelet activation, cell volume and phospholipid scrambling at the cell membrane was elucidated.
Materials and Methods Mice
All animal experiments were conducted according to the German law for the welfare of animals and were approved by the authorities of the state of Baden-Württemberg. Experiments were performed with blood platelets isolated from gene targeted mice lacking functional Ano6 (ano6-/-) and corresponding wild type mice (ano6+/+). The mice had free access to water and control chow (Ssniff, Soest, Germany). The generation of the mice has been described elsewhere [21].
Preparation of mouse platelets
Platelets were obtained from 10- to 12-week-old mice of either sex. The mice were anesthetized and 800 µl blood was drawn from the retro-orbital plexus into tubes with 200 µl acid-citrate-dextrose buffer before the mice were sacrificed [22]. Blood parameters (see table 1) were analyzed with a KX21-N automatic hematology analyzer (Sysmex). Platelet rich plasma (PRP) was obtained by centrifugation at 260 g for 5 minutes. Afterwards PRP was centrifuged at 640 g for 5 minutes to pellet the platelets. Where necessary apyrase (0.02 U/ml; Sigma-Aldrich) and prostaglandin I2 (0.5 µM; Calbiochem) were added to the PRP to prevent activation of platelets during isolation. After two washing steps the pellet of washed platelets was resuspended inmodified Tyrode-HEPES buffer (pH 7.4, supplemented with 1 mMCaCl2). Where indicated, thrombin (0.01 U/ml, Roche, Basel, Switzerland) or CRP (2 µg/ml or 5 µg/ml, kindly provided by R.Farndale, University of Cambridge, Cambridge, UK) were added [23]. Lower agonist concentrations have been used to activate the platelets, whereas higher agonist concentrations were required to trigger cell membrane scrambling.
Quantification of reactive oxygen species (ROS)
Oxidative stress was determined utilizing 2’,7’-dichlorodihydrofluorescein diacetate (DCFDA). Washed platelets were incubated for 15 minutes (37°C) with 0.01 U/ml thrombin and 2 µg/ml CRP, and washed two times with 350 µl Tyrode buffer after stimulation by agonists. They were subsequently stained with DCFDA (10 µM; Sigma, Schnelldorf, Germany) in Tyrode buffer at 37°C for 30 min and washed once in 150 µl Tyrode buffer. The DCFDA-loaded platelets were re-suspended in 200 µl Tyrode buffer and ROS-dependent fluorescence intensity was measured at an excitation wavelength of 488 nm and an emission wavelength of 530 nm on a BD FACS Calibur.
Cytosolic calcium measurements
For the measurement of the intracellular Ca2+ concentration the platelet preparation was washed once in Tyrode buffer (pH 7.4), stained with 3 µM Fluo-3AM (Biotinium, USA) in the same buffer and incubated at 37°C for 30 minutes. Following the indicated experimental treatment, fluorescence was measured at an excitation wavelength of 488 nm and an emission wavelength of 530 nm utilizing a BD FACS Calibur (BD Biosciences, Heidelberg, Germany) [24].
P-selectin and activated integrin abundance
Fluorophore-labeled antibodies were utilized for the detection of P-selectin expression (Wug.E9- FITC) and the active form of αIIbβ3 integrin (JON/A-PE). Washed mouse platelets (1x106) were suspended in modified Tyrode buffer (pH 7.4) containing 1 mM CaCl2 and antibodies (1:10 dilution)and subsequently stimulated with thrombin and CRP for the indicated time periods at room temperature (RT). The reaction was stopped by addition of PBS and the samples were immediately analyzed on a BD FACS Calibur.
Phosphatidylserine exposure and forward scatter
In order to determine phosphatidylserine exposure, the platelet preparation was centrifuged at 660 g for 5 minutes followed by washing once with Tyrode buffer (pH 7.4) with 1 mM CaCl2, staining with 1:20 dilution of Annexin-V-FITC (Mabtag, Germany) in Tyrode buffer (pH 7.4) with 2 mM CaCl2 and incubation at 37oC for 20 minutes. Annexin-V-binding reflecting surface exposure of phosphatidylserine was evaluated by flow cytometry utilizing a BD FACS Calibur. In parallel, the forward scatter (FSC) of the platelets was determined by flow cytometry as a measure of platelet size.
Platelet aggregation
Aggregation was determined utilizing flow cytometry as previously described [2]. To this end platelets were labeled with either CD9-APC or CD9-PE monoclonal antibodies (1:100 dilution, Abcam) for 15 minutes at room temperature. Following incubation, differently labeled samples were washed twice, mixed 1:1 and then pre-incubated at 37oC while shaking at 600 rpm for 10 min. Pre-incubated platelets were activated with thrombin or collagen related peptide at 37oC while shaking at 1000 rpm. At the indicated time points, samples were fixed by addition of 0.5% paraformaldehyde (Carl Roth, Germany) in phosphate-buffered saline. The fixed samples were measured utilizing a BD FACSCalibur (BD Biosciences, Heidelberg, Germany).
For quantification, a quadrant was set in the dot plot of respective channels on non-stimulated platelets. The appearance of double-colored events in the upper right quadrant (Q2) was quantified as percentage of total amount of labeled events (Q1+Q2+Q4) at every time point analyzed.
Statistical analysis
Data are provided as means ± SEM; n represents the number of independent experiments. All data were tested for significance using ANOVA with Tukey's test as post-test or unpaired student’s t-test as appropriate. Results with p<0.05 were considered statistically significant.
Results
The present study explored whether Ano6 modifies platelet activation and apoptosis.
To this end, murine platelets were isolated from gene targeted mice lacking functional Ano6 (ano6-/-) and corresponding wild type mice (ano6+/+). As listed in Table 1, the platelet count was slightly, but significantly (p<0.05) higher in ano6-/- than in ano6+/+ mice. Platelet volume, erythrocyte count, erythrocyte volume, hemoglobin concentration and white blood cell count were virtually identical in ano6-/- and ano6+/+ mice (Table 1).
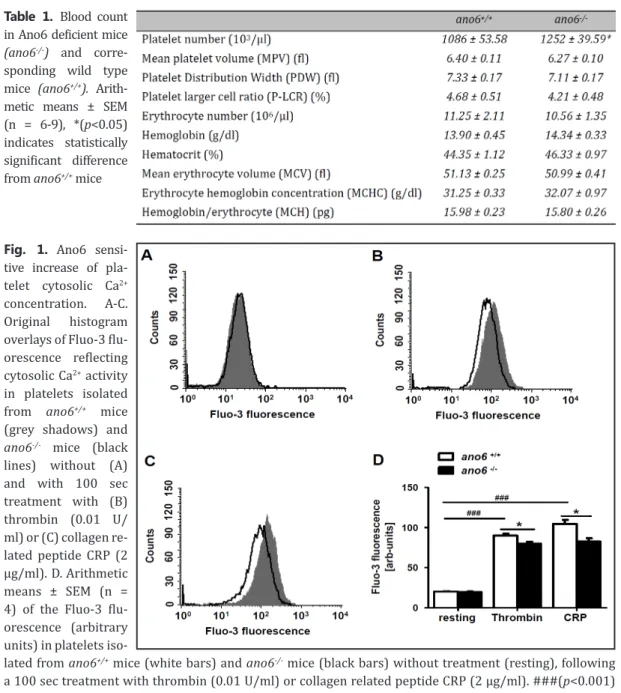
Fluo-3 fluorescence was employed to determine cytosolic Ca2+ activity ([Ca2+]i). Without treat- ment [Ca2+]i was similar in platelets isolated from ano6+/+ mice and ano6-/- mice (Fig. 1A, D). Activa- tion of platelets with thrombin (100 seconds, 0.01 U/ml) was followed by a significant increase of [Ca2+]i in both, ano6+/+ and ano6-/- platelets. The effect was, however, significantly less pronounced in ano6-/- platelets than in ano6+/+ platelets (Fig. 1B, D). Activation of the platelets with collagen re- lated peptide CRP (100 seconds, 2 µg/ml) was again followed by a significant increase of [Ca2+]i in
both, ano6+/+ and ano6-/- platelets. The effect was slightly but significantly smaller in ano6-/- platelets than in ano6+/+ platelets (Fig. 1C, D).
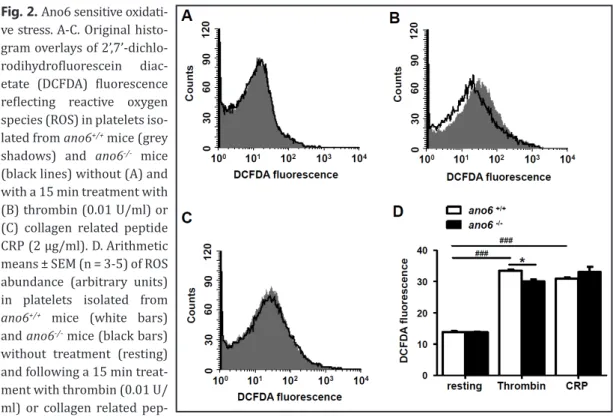
In order to test, whether Ano6 influences oxidative stress, the abundance of reactive oxygen species (ROS) was determined utilizing DCFDA fluorescence. Without treatment ROS was negligib- le in platelets of both genotypes (Fig. 2A, D). Activation of platelets with thrombin (15 min, 0.01 U/
ml) was followed by a significant increase of ROS in both, ano6+/+ and ano6-/- platelets, The effect was, however, significantly less pronounced in ano6-/- platelets than in ano6+/+ platelets (Fig. 2B, D).
Activation of the platelets with collagen related peptide CRP (15 min, 2 µg/ml) was again followed by a significant increase of ROS in both, ano6+/+ and ano6-/- platelets. The effect was, however, not significantly different between ano6-/- platelets and ano6+/+ platelets (Fig. 2C, D).
A next series of experiments addressed the impact of Ano6on platelet degranulation. The degranulation was apparent from P-selectin abundance at the platelet surface. As illustrated in Fig. 3,P-selectin abundance at the platelet surface was similarly low in untreated platelets Table 1. Blood count
in Ano6 deficient mice (ano6-/-) and corre- sponding wild type mice (ano6+/+). Arith- metic means ± SEM (n = 6-9), *(p<0.05) indicates statistically significant difference from ano6+/+ mice
Fig. 1. Ano6 sensi- tive increase of pla- telet cytosolic Ca2+
concentration. A-C.
Original histogram overlays of Fluo-3 flu- orescence reflecting cytosolic Ca2+ activity in platelets isolated from ano6+/+ mice (grey shadows) and ano6-/- mice (black lines) without (A) and with 100 sec treatment with (B) thrombin (0.01 U/
ml) or (C) collagen re- lated peptide CRP (2 µg/ml). D. Arithmetic means ± SEM (n = 4) of the Fluo-3 flu- orescence (arbitrary units) in platelets iso-
lated from ano6+/+ mice (white bars) and ano6-/- mice (black bars) without treatment (resting), following a 100 sec treatment with thrombin (0.01 U/ml) or collagen related peptide CRP (2 µg/ml). ###(p<0.001) indicates statistically significant difference from absence of thrombin and CRP, *(p<0.05) indicates statistically significant difference from ano6+/+ mice.
isolated from ano6+/+ mice and from ano6-/- mice. Activation of the platelets with thrombin (15 min, 0.01 U/ml) was followed by a significant increase of P-selectin abundance in both, ano6+/+ and Fig. 2. Ano6 sensitive oxidati-
ve stress.A-C. Original histo- gram overlays of 2’,7’-dichlo- rodihydrofluorescein diac- etate (DCFDA) fluorescence reflecting reactive oxygen species (ROS) in platelets iso- lated from ano6+/+ mice (grey shadows) and ano6-/- mice (black lines) without (A) and with a 15 min treatment with (B) thrombin (0.01 U/ml) or (C) collagen related peptide CRP (2 µg/ml). D. Arithmetic means ± SEM (n = 3-5) of ROS abundance (arbitrary units) in platelets isolated from ano6+/+ mice (white bars) and ano6-/- mice (black bars) without treatment (resting) and following a 15 min treat- ment with thrombin (0.01 U/
ml) or collagen related pep-
tide CRP (2 µg/ml). ###(p<0.001) indicates statistically significant difference from absence of thrombin and CRP, *(p<0.05) indicates statistically significant difference from ano6+/+ mice.
Fig. 3. Ano6 sensitive pla- telet degranulation. A-C. Ori- ginal histogram overlays of P-selectin related fluore- scence in platelets isolated from ano6+/+ mice (grey shadows) and ano6-/- mice (black lines) without (A) and with a 15 min treatment with (B) thrombin (0.01 U/
ml) or (C) collagen related peptide CRP (2 µg/ml). D.
Arithmetic means ± SEM (n = 4-7) of the P-selectin re- lated fluorescence (arbitrary units) in platelets isolated from ano6+/+ mice (white bars) and ano6-/- mice (black bars) without treatment (resting) and following a 15 min treatment with throm- bin (0.01 U/ml) or collagen related peptide CRP (2 µg/
ml). ###(p<0.001) indicates statistically significant difference from absence of thrombin and CRP, *(p<0.05) indicates statistically significant difference from ano6+/+ mice.
ano6-/- platelets. The effect tended to be slightly smaller in ano6-/- platelets than in ano6+/+ platelets, a difference, however, not reaching statistical significance (Fig. 3). Activation of the platelets with collagen related peptide CRP (15 min, 2 µg/ml) was again followed by a significant increase of P-selectin abundance in both, ano6+/+ and ano6-/- platelets. The effect was, however, significantly less pronounced in ano6-/- platelets than in ano6+/+ platelets (Fig. 3).
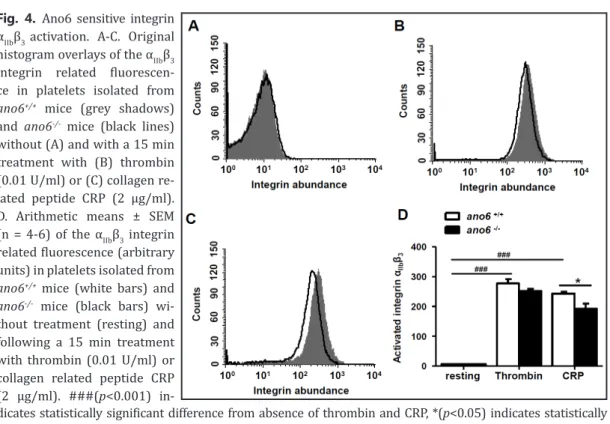
Further experiments addressed the impact of Ano6 on integrin αIIbβ3 activation.As illustra- ted in Fig. 4, the abundance of activatedintegrin αIIbβ3 at the platelet surface was similarly low in untreated platelets isolated from ano6+/+ mice and ano6-/- mice. Activation of the platelets with thrombin (15 min, 0.01 U/ml) was followed by a significant increase of the abundance of activated integrin αIIbβ3 in both, ano6+/+ and ano6-/- platelets. The effect tended to be smaller in ano6-/- pla- telets than in ano6+/+ platelets, a difference, however, not reaching statistical significance (Fig. 4).
Activation of the platelets with collagen related peptide CRP (15 min, 2 µg/ml) was again followed by a significant increase of integrin αIIbβ3 activation in both, ano6+/+ and ano6-/- platelets. The effect was significantly less pronounced in ano6-/- platelets than in ano6+/+ platelets (Fig. 4).
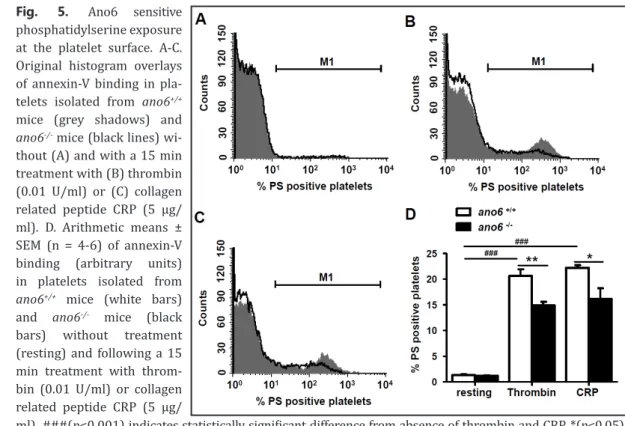
A further series of experiments explored the impact of Ano6 on platelet apoptosis, which is characterized by cell shrinkage and cell membrane scrambling with phosphatidylserine translocation to the platelet surface. Phosphatidylserine abundance was determined utilizing annexin-V-binding and platelet volume utilizing forward scatter. As illustrated in Fig. 5, annexin-V-binding was negligible in untreated platelets isolated from either genotype. Treatment with thrombin (0.01 U/ml) and CRP (5 µg/ml) was followed by an increase of annexin-V-binding in both, ano6+/+ and ano6-/- platelets. The effect was slightly, but significantly, blunted in ano6-/- platelets when compared to ano6+/+ platelets. As shown in Fig. 6, forward scatter was similar in untreated ano6-/- platelets and in untreated ano6+/+
platelets. Treatment with thrombin (15 min, 0.01 U/ml) was followed by a significant decrease of forward scatter in both, ano6+/+ and ano6-/- platelets. The effect was significantly less pronounced in ano6-/- platelets than in ano6+/+ platelets. Treatment with CRP (15 min, 5 µg/ml) was again followed by a significant decrease of forward scatter in both, ano6+/+ and ano6-/- platelets. The effect was again significantly less pronounced in ano6-/- platelets than in ano6+/+ platelets.
Fig. 4. Ano6 sensitive integrin αIIbβ3 activation. A-C. Original histogram overlays of the αIIbβ3 integrin related fluorescen- ce in platelets isolated from ano6+/+ mice (grey shadows) and ano6-/- mice (black lines) without (A) and with a 15 min treatment with (B) thrombin (0.01 U/ml) or (C) collagen re- lated peptide CRP (2 µg/ml).
D. Arithmetic means ± SEM (n = 4-6) of the αIIbβ3 integrin related fluorescence (arbitrary units) in platelets isolated from ano6+/+ mice (white bars) and ano6-/- mice (black bars) wi- thout treatment (resting) and following a 15 min treatment with thrombin (0.01 U/ml) or collagen related peptide CRP (2 µg/ml). ###(p<0.001) in-
dicates statistically significant difference from absence of thrombin and CRP, *(p<0.05) indicates statistically significant difference from ano6+/+ mice.
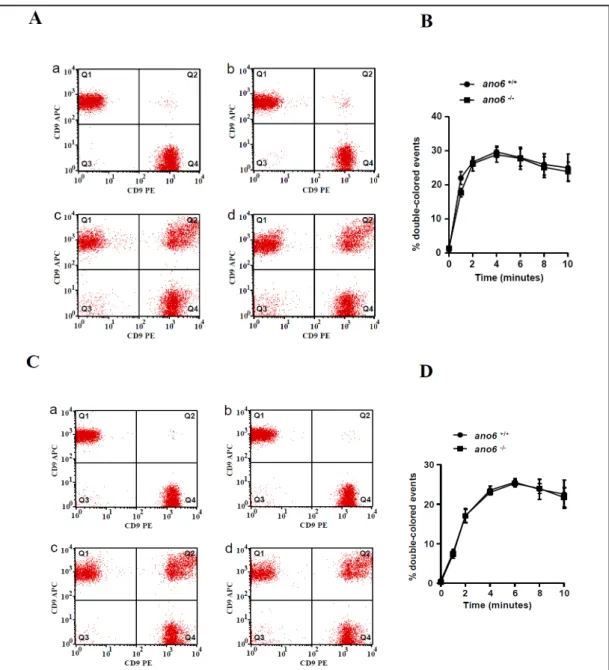
To elucidate the effect of Ano6 on platelet aggregation, platelets were labeled with two distinct dyes and the coincidence of the two dyes estimated by flow cytometry. As illustrated in Fig. 7, aggregation of resting platelets was similarly low in ano6+/+ and ano6-/- platelets, and Fig. 5. Ano6 sensitive
phosphatidylserine exposure at the platelet surface. A-C.
Original histogram overlays of annexin-V binding in pla- telets isolated from ano6+/+
mice (grey shadows) and ano6-/- mice (black lines) wi- thout (A) and with a 15 min treatment with (B) thrombin (0.01 U/ml) or (C) collagen related peptide CRP (5 µg/
ml). D. Arithmetic means ± SEM (n = 4-6) of annexin-V binding (arbitrary units) in platelets isolated from ano6+/+ mice (white bars) and ano6-/- mice (black bars) without treatment (resting) and following a 15 min treatment with throm- bin (0.01 U/ml) or collagen related peptide CRP (5 µg/
ml). ###(p<0.001) indicates statistically significant difference from absence of thrombin and CRP, *(p<0.05),
** (p<0.01) indicates statistically significant difference from ano6+/+ mice.
Fig. 6. Ano6 sensitive platelet forward scatter. A-C. Original histogram overlays of for- ward scatter in platelets iso- lated from ano6+/+ mice (grey shadows) and ano6-/- mice (black lines) without (A) and with a 15 min treatment with (B) thrombin (0.01 U/ml) or (C) collagen related peptide CRP (5 µg/ml). D. Arithmetic means ± SEM (n = 4-6) of the forward scatter of platelets isolated from ano6+/+ mice (white bars) and ano6-/- mice (black bars) without treat- ment (resting) and following a 15 min treatment with thrombin (0.01 U/ml) or col- lagen related peptide CRP (5 µg/ml). ###(p<0.001) indi- cates statistically significant difference from absence of
thrombin and CRP, *(p<0.05) indicates statistically significant difference from ano6+/+ mice.
significantly increased in a few minutes to similarly high levels in ano6+/+ and ano6-/- platelets following treatment with 0.005 U/ml thrombin or 2 µg/ml CRP.
Discussion
The present observations disclose a subtle but significant impact of Ano6 on platelet activation and apoptosis following thrombin or collagen related peptide (CRP) treatment.
Fig. 7. Ano6 insensitive platelet aggregation. A. Original dot blots reflecting platelet aggregation in platelets isolated from ano6+/+ mice (a, c) and ano6-/- mice (b, d), and subsequent treatment with 0.005 U/ml throm- bin in 0 min (a, b) and 10 min (c, d). B. Arithmetic means ± SEM (n = 4) of platelet aggregation in platelets isolated from ano6+/+ mice (black circles) and ano6-/- mice (black square) as a function of time after addition of thrombin (0.005 U/ml). C. Original dot blots reflecting platelet aggregation in platelets isolated from ano6+/+ mice (a, c) and ano6-/- mice (b, d), and subsequent treatment with collagen related peptide CRP (2 µg/ml) in 0 min (a, b) and 10 min (c, d). D. Arithmetic means ± SEM (n = 4) of platelet aggregation in platelets isolated from ano6+/+ mice (black circles) and ano6-/- mice (black square) as a function of time after addition of CRP (2 µg/ml).
Prior to activation, cytosolic Ca2+ activity ([Ca2+]i), reactive oxygen species (ROS), P-selectin abundance, αIIbβ3 integrin activation, phosphatidylserine exposure and cell volume were similar in platelets isolated from gene targeted mice lacking Ano6 (ano6-/-) and in platelets from corresponding wild-type mice (ano6+/+). Thrombin and CRP increased [Ca2+]i, as well as ROS abundance, and triggered platelet degranulation as well as αIIbβ3 integrin activation, effects less pronounced in ano6-/- than in ano6+/+ platelets. A key event in the activation of platelets is increase of [Ca2+]i [13], which triggers platelet degranulation, adhesion and aggregation thus supporting development of thrombosis [9].
Thrombin and CRP further trigger phosphatidylserine translocation and cell shrinkage, key events in apoptosis limiting the life span of circulating platelets [17]. The stimulation of platelet apoptosis may be similarly secondary to increase of [Ca2+]i [13], which is known to stimulate cell membrane phospholipid scrambling with phosphatidylserine translocation [8, 25-27]. According to the present observations, the phosphatidylserine exposure depends in part on the presence of Ano6. The thrombin and CRP induced cell shrinkage again depends in part on the presence of Ano6. It is conceivable that the Cl- channel function of Ano6 [1]
mediates Cl- exit and thus supports the loss of ions and osmotically obliged water [28].
A blunted Ca2+ increase was observed in ano6-/- platelets upon stimulation with thrombin and CRP. It is important to note that Ano6 has been described as a nonselective and Ca2+ permeable channel, which could be relevant for the present observations [29-31]. It should be pointed out, however that the scrambling defect in ano6-/- platelets is not due to defective Ca2+ influx through Ano6 [1]. It is possible that Ano6 affects intracellular Ca2+ signaling indirectly, as shown earlier for Ano1 [32].
Phosphatidylserine exposing platelets bind to and are engulfed by macrophages [33]. Phosphatidylserine at the platelet surface further stimulates coagulation and thus contributes to the triggering of hemostasis [34]. Phosphatidylserine exposure further stimulates thrombin formation and platelet pro-coagulant activity [25-27, 35].
The platelet number in blood was higher in ano6-/- mice than in ano6+/+mice. It is tempting to speculate that the decreased sensitivity of phosphatidylserine translocation to thrombin and CRP is followed by decreased clearance of phosphatidylserine exposing platelets from circulating blood.
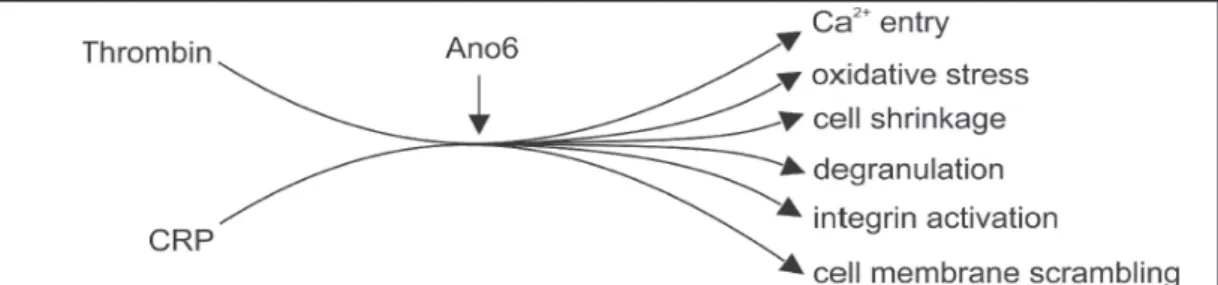
In conclusion, Ano6 contributes to both, platelet activation and platelet apoptosis (Fig. 8). Ano6 deficiency blunts the stimulating effect of thrombin and CRP on Ca2+ entry, platelet degranulation and integrin activation, phosphatidylserine translocation and platelet shrinkage. Thus, Ano6 is a novel element in the regulation of platelet function and survival.
Acknowledgements
No support received from individuals. The study was supported by the Deutsche Forschungsgemeinschaft (KFO 274) and Open Access Publishing Fund of Tuebingen University.
Fig. 8. Synopsis of Ano6 sensitive platelet functions.
Disclosure Statement
The authors of this manuscript state that they have no conflicts of interest to declare.
References
1 Kunzelmann K, Nilius B, Owsianik G, Schreiber R, Ousingsawat J, Sirianant L, Wanitchakool P, Bevers EM, Heemskerk JW: Molecular functions of anoctamin 6 (TMEM16F): a chloride channel, cation channel, or phospholipid scramblase? Pflugers Arch 2014;466:407-414.
2 De Cuyper IM, Meinders M, van de Vijver E, de Korte D, Porcelijn L, de Haas M, Eble JA, Seeger K, Rutella S, Pagliara D, Kuijpers TW, Verhoeven AJ, van den Berg TK, Gutierrez L: A novel flow cytometry-based platelet aggregation assay. Blood 2013;121:e70-80.
3 Ousingsawat J, Wanitchakool P, Kmit A, Romao AM, Jantarajit W, Schreiber R, Kunzelmann K: Anoctamin 6 mediates effects essential for innate immunity downstream of P2X7 receptors in macrophages. Nat Commun 2015;6:6245.
4 Picollo A, Malvezzi M, Accardi A: TMEM16 proteins: unknown structure and confusing functions. J Mol Biol 2015;427:94-105.
5 Scudieri P, Caci E, Venturini A, Sondo E, Pianigiani G, Marchetti C, Ravazzolo R, Pagani F, Galietta LJ: Ion channel and lipid scramblase activity associated with expression of TMEM16F/ANO6 isoforms. J Physiol 2015;593:3829-3848.
6 Gawaz M: Role of platelets in coronary thrombosis and reperfusion of ischemic myocardium. Cardiovasc Res 2004;61:498-511.
7 Borst O, Schmidt EM, Munzer P, Schonberger T, Towhid ST, Elvers M, Leibrock C, Schmid E, Eylenstein A, Kuhl D, May AE, Gawaz M, Lang F: The serum- and glucocorticoid-inducible kinase 1 (SGK1) influences platelet calcium signaling and function by regulation of Orai1 expression in megakaryocytes. Blood 2012;119:251-261.
8 Borst O, Munzer P, Gatidis S, Schmidt EM, Schonberger T, Schmid E, Towhid ST, Stellos K, Seizer P, May AE, Lang F, Gawaz M: The inflammatory chemokine CXC motif ligand 16 triggers platelet activation and adhesion via CXC motif receptor 6-dependent phosphatidylinositide 3-kinase/Akt signaling. Circ Res 2012;111:1297-1307.
9 Bergmeier W, Stefanini L: Novel molecules in calcium signaling in platelets. J Thromb Haemost 2009;7:S187- 190.
10 Varga-Szabo D, Braun A, Nieswandt B: Calcium signaling in platelets. J Thromb Haemost 2009;7:1057-1066.
11 Wang Y, Deng X, Gill DL: Calcium signaling by STIM and Orai: intimate coupling details revealed. Sci Signal 2010;3:e42.
12 Braun A, Varga-Szabo D, Kleinschnitz C, Pleines I, Bender M, Austinat M, Bosl M, Stoll G, Nieswandt B:
Orai1 (CRACM1) is the platelet SOC channel and essential for pathological thrombus formation. Blood 2009;113:2056-2063.
13 Varga-Szabo D, Braun A, Nieswandt B: STIM and Orai in platelet function. Cell Calcium 2011;50:270-278.
14 Shuttleworth TJ, Thompson JL, Mignen O: STIM1 and the noncapacitative ARC channels. Cell Calcium 2007;42:183-191.
15 Parekh AB: Store-operated CRAC channels: function in health and disease. Nat Rev Drug Discov 2010;9:399- 410.
16 Braun A, Vogtle T, Varga-Szabo D, Nieswandt B: STIM and Orai in hemostasis and thrombosis. Front Biosci (Landmark Ed) 2011;16:2144-2160.
17 Kile BT: The role of apoptosis in megakaryocytes and platelets. Br J Haematol 2014;165:217-226.
18 Bearer EL, Prakash JM, Li Z: Actin dynamics in platelets. Int Rev Cytol 2002;217:137-182.
19 Heemskerk JW, Bevers EM, Lindhout T: Platelet activation and blood coagulation. Thromb Haemost 2002;88:186-193.
20 Ouwehand WH, Bloodomics, Wellcome Trust Case Control C: Platelet genomics and the risk of atherothrombosis. J Thromb Haemost 2007;5:188-195.
21 Ehlen HW, Chinenkova M, Moser M, Munter HM, Krause Y, Gross S, Brachvogel B, Wuelling M, Kornak U, Vortkamp A: Inactivation of anoctamin-6/Tmem16f, a regulator of phosphatidylserine scrambling in osteoblasts, leads to decreased mineral deposition in skeletal tissues. J Bone Miner Res 2013;28:246-259.
22 Pelzl L, Fakhri H, Umbach AT, Gawaz M, Paulmichl M, Lang F: Sgk1 sensitive pendrin expression in murine platelets. Cell Physiol Biochem 2013;32:210-220.
23 Borst O, Walker B, Munzer P, Russo A, Schmid E, Faggio C, Bigalke B, Laufer S, Gawaz M, Lang F: Skepinone-L, a novel potent and highly selective inhibitor of p38 MAP kinase, effectively impairs platelet activation and thrombus formation. Cell Physiol Biochem 2013;31:914-924.
24 Towhid ST, Schmidt EM, Tolios A, Munzer P, Schmid E, Borst O, Gawaz M, Stegmann E, Lang F: Stimulation of platelet death by vancomycin. Cell Physiol Biochem 2013;31:102-112.
25 Harper MT, Poole AW: Store-operated calcium entry and non-capacitative calcium entry have distinct roles in thrombin-induced calcium signalling in human platelets. Cell Calcium 2011;50:351-358.
26 Mahaut-Smith MP: A role for platelet TRPC channels in the Ca2+ response that induces procoagulant activity.
Sci Signal 2013;6:pe23.
27 Mushtaq M, Nam TS, Kim UH: Critical role for CD38-mediated Ca2+ signaling in thrombin-induced procoagulant activity of mouse platelets and hemostasis. J Biol Chem 2011;286:12952-12958.
28 Lang F, Hoffmann EK: Role of ion transport in control of apoptotic cell death. Compr Physiol 2012;2:2037- 2061.
29 Jin X, Shah S, Liu Y, Zhang H, Lees M, Fu Z, Lippiat JD, Beech DJ, Sivaprasadarao A, Baldwin SA, Zhang H, Gamper N: Activation of the Cl- channel ANO1 by localized calcium signals in nociceptive sensory neurons requires coupling with the IP3 receptor. Sci Signal 2013;6:ra73.
30 Yang H, Kim A, David T, Palmer D, Jin T, Tien J, Huang F, Cheng T, Coughlin SR, Jan YN, Jan LY: TMEM16F forms a Ca2+-activated cation channel required for lipid scrambling in platelets during blood coagulation. Cell 2012;151:111-122.
31 Yu K, Whitlock JM, Lee K, Ortlund EA, Yuan Cui Y, Hartzell HC: Identification of a lipid scrambling domain in ANO6/TMEM16F. Elife 2015;4:
32 Tian Y, Schreiber R, Kunzelmann K: Anoctamins are a family of Ca2+-activated Cl- channels. J Cell Sci 2012;125:4991-4998.
33 Badlou BA, Wu YP, Smid WM, Akkerman JW: Platelet binding and phagocytosis by macrophages. Transfusion 2006;46:1432-1443.
34 Lhermusier T, Chap H, Payrastre B: Platelet membrane phospholipid asymmetry: from the characterization of a scramblase activity to the identification of an essential protein mutated in Scott syndrome. J Thromb Haemost 2011;9:1883-1891.
35 Wolfs JL, Comfurius P, Rasmussen JT, Keuren JF, Lindhout T, Zwaal RF, Bevers EM: Activated scramblase and inhibited aminophospholipid translocase cause phosphatidylserine exposure in a distinct platelet fraction. Cell Mol Life Sci 2005;62:1514-1525.