Materials and Corrosion
Relevant Thermochemical Processes in Biomass Fired Power Plants
Wolfgang Spiegel, Marie Kaiser, Gabriele Magel and Werner Schmidl
1. Heavy metals ...457
2. Chlorine and bromine ...459
3. Alkalis and earth alkalis ...461
4. Sulfur ...463
5. Recommendation to the market ...463
6. References ...464 The experiences gained during the last decades with fouling and corrosion in power plants fired with difficult fuels like waste derived from household and/or industry strongly influence the design of the boilers and the materials used. It is a lessons lear- ned situation, at least for fuels dominated by household derived waste. Conservative steam parameters as well as high quality and high performance materials are commonly accepted as necessary. [1, 2, 3, 4]
Contrary to this, power plants fired with biomass derived fuels are regarded as less affected by problems with fouling and corrosion. Aims for higher efficiency, lower investment, cheaper materials and simpler design seem to be appropriate. This is a situation for lessons to be learned.
According to the experience of the authors, various kinds of biomass show – from a thermochemical point of view – a corrosion behaviour similar to waste and should be treated in power plants with a design and materials suitable for waste.
The main relevant chemical components, which are responsible for a thermochemical classification of biomass as a kind of waste, are heavy metals (lead and zinc), chlorine, bromine, alkalis (potassium and sodium), earth alkalis (calcium and magnesium) and with a specific view sulphur. The amount and the proportions of these components in the fuel define, whether a potential for high temperature corrosion and strong fouling is given. Some aspects about the mechanisms and effects of these chemical components in biomass fuels in terms of fouling and corrosion will be regarded in the following text.
1. Heavy metals
Lead, zinc, copper, tin and other heavy metal elements tend to be mobilised from the fuel into the flue gas stream, regardless of whether the firing conditions are oxidising, reducing, alternating or stepwise and regardless of whether the type of firing is like
Materials and Corrosion
grate, BFB (bubbling fluidized-bed boiler), CFB (circulating fluidized-bed boiler) or gasification. The compounds of heavy metals given in the biomass fuel are not of strong relevance as well. Whether it is an alloy or a carbonate or another inorganic or organic compound or a mixture of these does not change the general tendency of mobilisation into the flue gas. Only specific oxides and silicates may show a different behaviour.
Typically the grain size of heavy metal compounds in biomass fuels is very fine, i.e.
colour pigments, which also support the transfer into the flue gas.
In the case of chlorine or bromine beeing available in the fuel, heavy metals may form chlorides/bromides. Due to the thermodynamic characteristics of such salts, a strong and selective enrichment on boiler tubes is likely, especially near the tube surface (first several 100 microns inside the fouling and/or inside the corrosion products). This process is called desublimation.
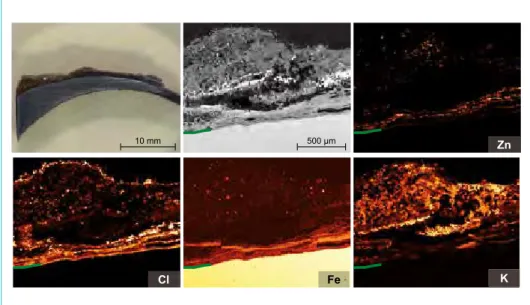
Figure 1: With the scanning electron microscope, the structure of the fouling can be seen at the dry polished section. In the element mapping, the distribution of the different elements can be studied (yellow: high amount; black: low amount; the green line marks the steel surface). Finding: High temperature chlorine corrosion on boiler tubes is mainly induced by zinc-potassium-chloride. Due to desublimation, high enrichment of zink near to the cold tube surface occurred.
The particles formed by desublimation are in the range of aerosols, smaller than 1 mi- cron. After the deposition and formation of such fouling, the salts may modify as a kind of solid-solid reaction (formation of mixed salts) or solid-gaseous reaction (sulphation).
According to these reactions, new salt mixtures are formed. Due to different melting temperatures of these different heavy metal salts and due to systematic and fluctuating changes of the temperature distribution within the fouling (depending on complex influencing factors like heat flux, heat transfer, thickness of fouling, online cleaning
Materials and Corrosion
etc.), the salts will melt within the fouling. Formation of salt melts, crystallisation of salt minerals and evaporation of salts will happen continuously. The formation of corrosion products at the corrosion front can influence and intensify these processes further. [1, 3]
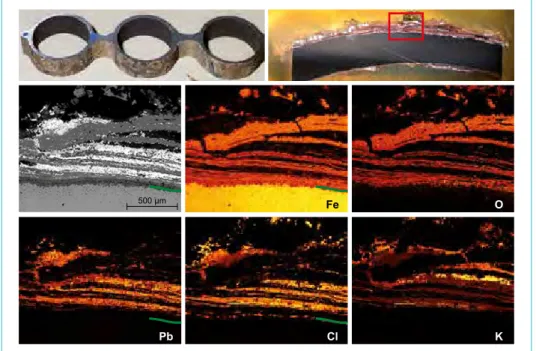
Figure 2: Section of an evaporator wall (250 °C), embedded, polished and investigated with SEM- EDX. Finding: Lead chloride (in mixture with potassium) is deposited in the cold caverns between the iron oxide corrosion products near to the tube surface.
Even small loads of heavy metals in the biomass fuel may have a strong impact on cor- rosion due to the strong enrichment potential of heavy metal chlorides on boiler tubes.
2. Chlorine and bromine
Chlorine in the biomass fuel may be present in a high content due to waste-like minor components (plastics) in the fuel. Other factors, such as a natural/technical impact like road salt, sea salt, fertilizer or sticking soil, can also be responsible for higher chlorine content. Nevertheless, in most cases the whole load of chlorine in the fuel will be activated into the flue gas stream. Reactions with heavy metals as well as alkalis and earth alkalis are typical. This formation of chlorides happens in the gaseous state and therefore chlorides may influence the formation of fouling until the saturation temperature is reached by general or local cooling of the flue gas in the boiler. At such temperature conditions a fog of a specific salt is formed at a specific position within the flue gas. The consequence is a strong enrichment of this specific salt within the fouling at that specific part of the flue gas path. The temperature milieu and position of the fog depend on the load of the specific salt. Again a complex and dynamic process is given, which is related to the fuel, the firing and the cooling conditions in the boiler. [2, 4]
Fe
Cl K
Pb
500 µm O
Materials and Corrosion
Figure 3: Preparation of a superheater tube (430 °C). Finding: Formation of iron chloride at the corrosion front. An intercrystalline attack of the alloy can be observed. The upper left picture shows the roughened surface of the tube after cleaning.
Bromine typically is part of waste-like minor components derived from flame retar- dant materials. But a natural impact from sea salt is possible as well, as documented for specific peats. Bromine and chlorine components show a similar behaviour during incineration with high transfer rates into the flue gas. In terms of high tempe- rature corrosion, bromine is more aggressive than chlorine, predominantly forming pitting.
The load of chlorine and bromine in the fuel directly influences the load of chlorides and bromides in the flue gas. The chlorides and bromides are usually bound to alkalis, earth alkalis,heavy metals or ammonium. The proportions of these salts in relation to HCl and HBr differ widely. At the cold end of the boiler, the load of chlorine bound as HCl and bromine bound as HBr in the flue gas typically dominates the load of chlorine bound as chloride and bromine bound as bromide.
The load of chlorine in the fuel is of major importance for high temperature corrosion.
In comparison the load of bromine is of minor importance, due to a typically much lower load (more than ten times lower). However, the tendency of bromine to increase pitting has to be kept in mind.
The reduction of chlorine (and bromine) within the biomass fuel before incineration is highly recommended.
Materials and Corrosion
3. Alkalis and earth alkalis
Biomass typically contains a high load of potassium, a biological key-element. Depen- ding on the type of biomass, the load of potassium varies in a wide range. The load of sodium is lower in relation to potassium for most of biomass fuels. [4]
If chlorine (analogous bromine) is available in the fuel, the formation of potassium chloride (bromide) during firing is likely. If there are heavy metals present simulta- neously, the melting temperature of the given salt may be lowered down close to the temperature that is given at the tube surface. This means, strong corrosion problems may occur at the evaporation tubes. If there are no heavy metal chlorides given, the corrosion and fouling problems are related to the hotter parts of the boiler only, the superheater.
Calcium becomes part of biomass fuel due to sticking soil or other waste-like compo- nents like paper and plastic. If the calcium containing mineral is calcium carbonate, the contribution of calcium to corrosion and fouling may be of relevance. In this case calcination reactions followed by reaction with SO2/HCl cause the formation of calcium chloride or calcium sulphate, which may directly or indirectly contribute to corrosion.
If the calcium component in the fuel is calcium silicate, the relevance for corrosion and fouling is much lower.
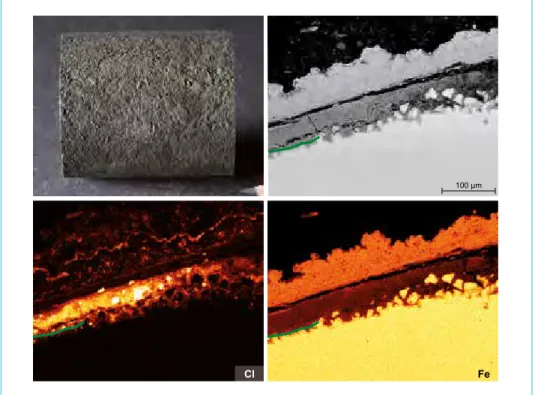
Figure 4:
Preparation of an evaporator tube (approximately 150 °C).
Finding: High enrichment of chlorine and bromine at the cor- rosion front. Due to hygroscopy this salts are mobilized from the corrosion front to the cutting surface of the tube during prepa- ration. The cleaned tube shows local pitting.
Materials and Corrosion
Figure 5:
Preparation of a superheater tube (approximately 430 °C).
Finding: In this case, potassium chloride causes the main corro- sive attack on the superheater tube. The presence of sulphides at the corrosion front indicates a very low oxygen partial pressure.
Figure 6:
Preparation of a superheater tube (approximately 475 °C).
Finding: Calcium chloride is embedded in the corrosion layer.
Due to the hygroscopic behavi- our of this salt, the salt reacts with the humidity of the air and droplets occur on the surface of the polished section.
Materials and Corrosion
4. Sulfur
A high load of sulphur in the fuel typically limits high temperature corrosion problems, however may force fouling problems, as the sulphur species are able to sulphate the chlorides. Doing this completely, the melting temperature of the salts rises distinctly and no salt melts are given close to the tube surface anymore.
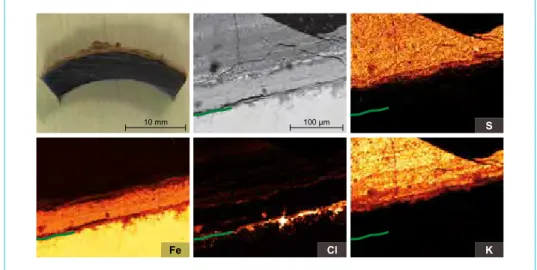
Figure 7: Preparation of a superheater tube (approximately 400 °C). Finding: Fouling built up mainly of potassium sulphate.
In general, if there are no sulphur bearing species given in the flue gas after the boiler, which means no sulphur components to be cleaned off from the flue gas, an addition of sulphur species to the biomass fuel (additive) may be of interest.
5. Recommendation to the market
Unknown or modified types of biomass fuel should be tested in relation to its ther- mochemical behaviour before it is used in firing processes with heat recovery systems (boiler). Suitable testing facilities are laboratory scaled batch reactors, for example with a load of 10 kilograms of fuel to be fired, in combination with specific probe measurements to identify the kind of particles and resulting salt loads in the flue gas.
Situations like planning a new power plant fired with biomass or a retrofit of an exis- ting power plant or a change from fossil fuel to biomass fuel should be accompanied during the first 10,000 hours of operation intensively by online measures with probes to quickly identify the risks of corrosion and fouling and to find out optimisation potentials. These probes may be temperature range probes, grid probes or ASP, as developed by CheMin. [1, 2, 3, 4]
Do not believe biomass is a harmless fuel in terms of corrosion and fouling.
Materials and Corrosion
6. References
[1] Kaiser, M.; Spiegel, W. : Thermochemische Prozesse verstehen und verbessern. In: Beckmann, M.; Hurtado, A. (Eds.): Kraftwerkstechnik 2017 – Strategien, Anlagentechnik und Betrieb, pp. 329-341. Available on www.chemin.de.
[2] Magel, G.: Get to Know the Corrosion Mechanisms in Waste-to-Energy Plants. In: Thomé- Kozmiensky, K. J.; Thiel, S.; Thomé-Kozmiensky, E.; Winter, F.; Juchelková, D. (Eds.): Waste Management, Volume 7, Waste-to-Energy. Neuruppin: TK Verlag Karl Thomé-Kozmiensky, 2017, pp. 501-513. Available on www.chemin.de.
[3] Müller, W.; Kaiser, M.; Schneider, D.; Herzog, T.; Magel, G.; Spiegel, W.: Korrosion durch Altholzeinsatz in Biomassekraftwerken. In: Born, M. (Ed.): Dampferzeugerkorrosi- on 2013, Freiberg: SAXONIA Standortentwicklungs- und verwaltungsgesellschaft mbH, pp. 177-195. Available on www.chemin.de.
[4] Müller, W.; Schneider, D.; Kaiser, M.; Brell, J.; Spiegel, W.; Pohl, M.: Fuel leaflets for the prevention of negative impact on the boiler from minor fuel constituents. VGB PowerTech, Vol. 7/2014, pp. 76-81. Available on www.chemin.de.
Contact Person
Dr. rer. nat. Wolfgang Spiegel CheMin GmbH
Managing director Am Mittleren Moos 46 A 86167 Augsburg
GERMANY
Phone: 0049 - 821 - 74 83 90 Email: chemin@chemin.de
Bibliografische Information der Deutschen Nationalbibliothek Die Deutsche Nationalbibliothek verzeichnet diese Publikation in der Deutschen Nationalbibliografie; detaillierte bibliografische Daten sind im Internet über http://dnb.dnb.de abrufbar
Thiel, S.; Thomé-Kozmiensky, E.; Winter, F.; Juchelková, D. (Eds.):
Waste Management, Volume 8 – Waste-to-Energy –
ISBN 978-3-944310-42-8 Thomé-Kozmiensky Verlag GmbH
Copyright: Elisabeth Thomé-Kozmiensky, M.Sc., Dr.-Ing. Stephanie Thiel All rights reserved
Publisher: Thomé-Kozmiensky Verlag GmbH • Neuruppin 2018 Editorial office: Dr.-Ing. Stephanie Thiel, Dr.-Ing. Olaf Holm,
Elisabeth Thomé-Kozmiensky, M.Sc.
Layout: Janin Burbott-Seidel, Ginette Teske, Roland Richter, Cordula Müller, Sarah Pietsch, Gabi Spiegel, Lena Bischkopf
Printing: Universal Medien GmbH, Munich
This work is protected by copyright. The rights founded by this, particularly those of translation, reprinting, lecturing, extraction of illustrations and tables, broadcasting, micro- filming or reproduction by other means and storing in a retrieval system, remain reserved, even for exploitation only of excerpts. Reproduction of this work or of part of this work, also in individual cases, is only permissible within the limits of the legal provisions of the copyright law of the Federal Republic of Germany from 9 September 1965 in the currently valid revision. There is a fundamental duty to pay for this. Infringements are subject to the penal provisions of the copyright law.
The repeating of commonly used names, trade names, goods descriptions etc. in this work does not permit, even without specific mention, the assumption that such names are to be considered free under the terms of the law concerning goods descriptions and trade mark protection and can thus be used by anyone.
Should reference be made in this work, directly or indirectly, to laws, regulations or guide- lines, e.g. DIN, VDI, VDE, VGB, or these are quoted from, then the publisher cannot ac- cept any guarantee for correctness, completeness or currency. It is recommended to refer to the complete regulations or guidelines in their currently valid versions if required for ones own work.