Ζ. Kristallogr. NCS 216 (2001) 17
> by Oldenbourg Wissenschaftsverlag, München
C r y s t a l s t r u c t u r e o f c a e s i u m μ - ο χ ο η κ Ι ί β Κ Π ^ ί β β ί Ι ^ Ι β , C s ö t N i S i z O s ]
J. Hansing, P. Amann and A. Möller*
Universität zu Köln. Institut f ü r A n o r g a n i s c h e C h e m i e . G r e i n s t r a ß e 6. D - 5 0 9 3 9 Köln. G e r m a n y
R e c e i v e d J u l v 14. 2 0 0 0 . C S D - N o . 4 0 9 5 0 6
Abstract
Cs
6Ni0
8Si2, triclinic, P\ (No. 2), a = 6.892(2) A,
b = 7.043(2) Ä, c = 8.069(2) Α, α = 87.36(3)°, β = 88.79(3)°, γ = 70.08(3)°, V= 367.8 A
3, Z = 1, Rp(F) = 0.028,
wR
ref(F
2) = 0.054, T= 293 K.
trans-edges. Typical interatomic distances are found for [S1O4], though due to the edge sharing arrangement with [N1O4], consid- erable distortion of the bridging angle is observed, e.g.
Z 0 ( l ) - S i - 0 ( 2 ) = 98.2(3)°. The cell parameters obtained by powder diffraction refinement are: a = 6.881(1) k\b = 7.045(2) A; c = 8.063(2) Α; α = 87.38(1)°; β = 88.79(2)°; γ = 70.01(3)°.
Table 1. Data collection and handling.
Crystal: transparent green-blue, irregular, size 0.100 χ 0.075 χ 0.125 mm Wavelength: Mo Ka radiation (0.71073 A)
μ:
161.30 cm"1Diffractometer, scan mode: STOE IPDS, ψ
20max • 53.98°
WAdmeasured, N(hkl)mjque: 4411,1497 Criterion for /obs, N(hkl)gC. U s > 2 a(Iobs), 1054 N(param InUnca: 80
Programs: SHELXS-97 [6], SHELXL-97 [7], DIAMOND [8], Win XPOW [9]
Acknowledgment. Financial support by the Deutsche Forschungs- gemeinschaft is gratefully acknowledged.
Source of material
Cs6[NiSi20e] could not be synthesized by annealing intimate mixtures of the binary compounds, but can be obtained via redox reactions with Ni containers. Oxidizing agents are Ag20, CdO, CU2O and CuO, respectively, being reduced to the metal in the presence of CS2O and S1O2 (typical molar ratios 3.3:2) [ 1,2]. The title compound can be obtained under inert gas atmosphere in sealed Ni containers at 773 K. This type of reaction has been de- scribed previously by Hoppe et al. [3,4].
Discussion
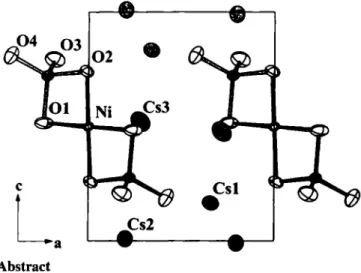
Cs6[NiSi20e] crystallizes isotypic to K6[CuSi20e] [5]. The crys- tal structure contains isolated complex anions [NiSi
20
8]
6" with Ni
2+in nearly square-planar coordination (<i(Ni—O) = 1.982(6) A and 1.971(5) A). Connection to [S1O4] tetrahedra occurs via
Table 2. Atomic coordinates and displacement parameters (in Ä2).References
1. Hansing, J.: Ternäre Oxide und Silicate mit Übergangsmetallen (insbesondere Cobalt), Dissertation, Universität zu Köln, Germany 1999.
2. Amann, P.: Unpublished results, Universität zu Köln, Germany 1999.
3. Bernhardt, F.; Hoppe, R.: Das erste Oxoferrat(I): Zur Konstitution von K3[Fe02] und Κ3[Νίθ2], Z. Anorg. Allg. Chern. 619 (1993) 969-975.
4. Burow, W.; Birx, J.; Bernhardt, F.; Hoppe, R.: Zur Existenz polynärer Oxide der Alkalimetalle mit einwertigem Cobalt bzw. Nickel, Z. Anorg.
Allg. Chem. 619 (1993) 923-933.
5. Möller, Α.: Synthese, Kristallstrukturen und Absorptionsspektren der neuen "Cupriosilicate" KetCuSijOs] und Rb4[CuSi2(>7], Z. Anorg. Allg.
Chem. 623 (1997) 1685-1692.
6. Sheldrick, G. M.: SHELXS-97-2, Program for the Solution of Crystal Structures. University of Göttingen, Germany 1997.
7. Sheldrick, G. M.: SHELXL-97-2, Program for Crystal Structure Refine- ment. University of Göttingen, Germany 1997.
8. Brandenburg, K.: Diamond (Version 2.1a). Crystal Impact GbR, Germany 1996-1999.
9. Stoe & Cie: Win XPOW (Version 1.06), Darmstadt, Germany 1999.
Atom Site χ y ζ U11 U22 t/33 U12 U13 i/23 Cs(l) 2i -0.33900(9) 0.8293(1) 0.16599(6) 0.0184(3) 0.0189(3) 0.0144(3) 0.0032(2) -0.0019(2) -0.0005(2) Cs(2) 2/ -0.20190(9) 1.29748(9) -0.00931(6) 0.0159(3) 0.0158(3) 0.0197(3) -0.0060(2) -0.0001(2) 0.0002(2) Cs(3) 2/ -0.2815(1) 1.2075(1) 0.47804(7) 0.0260(4) 0.0280(4) 0.0276(3) -0.0151(3) -0.0072(3) 0.0074(3)
Ni i s 0 1/2 1/2 0.0075(7) 0.0056(7) 0.0052(6) -0.0006(6) 0.0011(5) -0.0005(5)
Si 2/ -0.2225(4) 0.7391(4) -0.2767(2) 0.010(1) 0.008(1) 0.0072(9) 0.0021(9) 0.0004(8) -0.0013(8) O(l) 2/ -0.242(1) 0.7512(9) 0.5162(6) 0.024(4) 0.015(3) 0.010(3) 0.005(3) 0.000(2) -0.003(2) 0(2) 21 0.0118(9) 0.4676(9) 0.2582(6) 0.015(3) 0.019(3) 0.010(2) 0.002(3) -0.003(2) 0.001(2) 0(3) 2i -0.184(1) 0.936(1) -0.2069(7) 0.027(4) 0.017(4) 0.017(3) -0.003(3) -0.004(3) -0.006(3) 0(4) 2/ -0.422(1) 0.703(1) -0.1887(7) 0.015(3) 0.020(4) 0.022(3) -0.006(3) 0.007(2) 0.000(3)