Research Collection
Doctoral Thesis
Characterization of inflammatory responses in mouse models of asthma and wound healing
Author(s):
Pohlmeier, Lea Publication Date:
2021
Permanent Link:
https://doi.org/10.3929/ethz-b-000479843
Rights / License:
In Copyright - Non-Commercial Use Permitted
This page was generated automatically upon download from the ETH Zurich Research Collection. For more information please consult the Terms of use.
DISS. ETH NO. 27415
Characterization of inflammatory responses in mouse models of asthma and wound healing
A thesis submitted to attain the degree of DOCTOR OF SCIENCES of ETH ZURICH
(Dr. sc. ETH Zurich)
Presented by
LEA MARIA POHLMEIER MSc ETH Zurich
Born on 08.09.1989 Citizen of Germany
accepted on the recommendation of:
Prof. Dr. Manfred Kopf; examiner Prof. Dr. Burkhard Becher; co-examiner Prof. Dr. Manfred Claassen; co-examiner
Dr. Luigi Tortola; co-examiner
2021
This thesis is dedicated to my parents;
Anette and Winfried Pohlmeier and to my sister Mara Pohlmeier.
Thank you for your love and support.
Table of Contents
1 SUMMARY ... 5
2 ZUSAMMENFASSUNG ... 7
3 INTRODUCTION ... 11
3.1 IMMUNE SYSTEM ... 11
3.2 INNATE IMMUNITY ... 11
3.2.1 Dendritic cells ... 13
3.2.1.1 Conventional DCs ... 14
3.2.1.2 Plasmacytoid DCs ... 16
3.2.1.3 Monocyte-derived DCs ... 16
3.2.2 Eosinophils ... 16
3.2.3 Innate lymphoid cells ... 17
3.2.4 Macrophages ... 17
3.2.5 Mast cells ... 18
3.2.5.1 Mouse models of mast cell deficiency ... 20
3.2.6 Monocytes ... 21
3.2.7 Natural killer cells ... 21
3.2.8 Neutrophils ... 21
3.3 ADAPTIVE IMMUNITY ... 22
3.3.1 T cells ... 22
3.3.1.1 CD4+ T cells ... 23
3.3.1.2 CD8+ T cells ... 28
3.3.1.3 gd T cells ... 29
3.3.2 B cells ... 30
3.4 ASTHMA ... 30
3.4.1 Murine models of allergic airway inflammation ... 32
4 RESULTS ... 35
4.1 COMPARISON OF AIRWAY INFLAMMATION AND T CELL RESPONSES IN DIFFERENT ACUTE AND CHRONIC MOUSE ASTHMA MODELS 35 4.2 COMPARATIVE ANALYSIS OF MAST CELL DEFICIENCY IN ACUTE AND CHRONIC MURINE ASTHMA MODELS ... 69
4.3 COMPREHENSIVE CHARACTERIZATION OF MYELOID CELLS DURING WOUND HEALING IN HEALTHY AND HEALING-IMPAIRED DIABETIC MICE ... 93
5 GENERAL DISCUSSION ... 123
6 REFERENCES ... 127
7 APPENDIX ... 135
7.1 ABBREVIATIONS ... 135 7.2 CURRICULUM VITAE ... 137 8 ACKNOWLEDGEMENTS ... 139
5
1 Summary
The immune system of higher animals is a complex network of organs, cells and molecules.
As such, focusing on individual aspects of this network risks overlooking or possibly neglecting the global interconnections. The goal of this thesis is to take a step back and analyze the immune response in a more comprehensive manner. In order to achieve this goal, we focus on two quite different types of immune response: (i) the immune response to allergic airway inflammation and (ii) the immune response in wound healing.
The first type of immune responses we study here are different models of allergic airway inflammation. A variety of asthma models have been developed in recent decades with the goal of mimicking certain features of human asthma. However, while many of these models mimic different aspects of the human disease, they exhibit different compositions of cells infiltrating the airways or the lung tissue. In addition to cellular infiltration, the composition and amount of cytokines produced by these cells play a fundamental role in the development and severity of different types of asthma. For this purpose, we use different pathways and regimes of sensitization. These sensitizations were done via the peritoneum, skin or lungs, followed by challenges via the lungs. We also used different allergens (i.e. ovalbumin and house dust mite extract). In addition to trying to understand the basis of the different models of acute and chronic asthma, we are also trying to gain deeper insight into the influence of the genetic background. BALB/c mice are still widely used in animal research, although C57BL/6 mice are the predominantly used mouse strain. Because BALB/c mice show a Th2 response when infected with Leishmania major, they are often considered "Th2 responders,"
which implies that they should show a more severe phenotype in allergic airway inflammation models. However, our results show that BALB/c mice exhibit consistently reduced infiltration of leukocytes into the airways. This is mainly due to their reduced eosinophilia when compared to C57BL/6 mice.
Mast cells (MCs) are considered to be key players in human asthma and it has been shown, that they release important inflammatory mediators after allergen exposure. However, and surprisingly, their role in animal models remains controversial. This is largely due to the lack of specific MC-deficient mouse models that do not exhibit MC-independent effects. In our thesis, we use Cpa3Cre/+ mice with functional Kit signaling that are nevertheless MC deficient.
These animals show a pronounced deficiency of MCs but are not otherwise affected (except for a reduction in basophils) by effects elsewhere. Here, we use the above models to resolve the aforementioned controversies surrounding the role of MCs in murine asthma models. We are able to show that the absence of MCs has no effect on the severity of allergic airway inflammation in any of the models tested.
The second type of immune response that we characterize in more detail in this thesis is the immune response during wound healing. The interaction of different lymphoid and myeloid cell populations is necessary to protect the host from the invasion of potential pathogens into a wound and to support the epithelial cells to close the lesion. In addition to the characterization of infiltrating myeloid cells, we also study their kinetics during the healing process. This analysis was performed in healthy and healing-impaired diabetic mice. In early wounds, diabetic mice show reduced numbers and percentages of macrophages and monocyte-derived dendritic cells (moDCs). When compared to their healthy counterparts, the number of Langerhans cells, conventional DCs and eosinophils were also reduced throughout the healing process. In contrast, during the late phases of wound healing, the healing- impaired diabetic mice showed a significantly higher proportion of immune cells. This could be attributed to the persistence of neutrophils, macrophages, and monocytes. Analyzing the concerted actions of immune cells during wound healing, especially in the context of impaired healing, may help to understand chronic wounds and offer new possibilities for wound treatment.
Overall, the work presented in this dissertation highlights the composition and number of immune cells that infiltrate the airways and lung tissue in the context of allergic airway inflammation. Furthermore, this dissertation contributes to a more accurate description of the infiltration as well as the kinetics of myeloid cells during the different stages of wound healing.
7
2 Zusammenfassung
Das Immunsystem höherer Tiere ist ein komplexes Netzwerk aus Organen, Zellen und Molekülen. Die Fokussierung auf einzelne Aspekte dieses Netzwerks birgt jedoch die Gefahr, die globalen Zusammenhänge zu übersehen oder gegebenenfalls zu vernachlässigen. Das Ziel dieser Arbeit ist es, einen Schritt zurückzutreten und die Immunantwort auf eine umfassendere Weise zu analysieren. Um dieses Ziel zu erreichen, fokussieren wir uns in dieser Arbeit auf zwei sehr unterschiedliche Arten der Immunantwort: (i) die Immunantwort auf allergische Atemwegsentzündungen und (ii) die Immunantwort bei Wundheilungen.
Die erste Art von Immunantworten, die wir untersuchen, sind verschiedene Modelle der allergischen Atemwegsentzündung. So wurden in den letzten Jahrzehnten eine Vielzahl von Asthmamodellen entwickelt, mit dem Ziel bestimmte Merkmale menschlichen Asthmas zu imitieren. Während viele dieser Modelle verschiedene Aspekte der menschlichen Erkrankung nachbilden, zeigen sie jedoch unterschiedliche Zusammensetzungen an Zellen, die in die Atemwege oder in das Lungengewebe infiltrieren. Neben der zellulären Infiltration spielen auch die Zusammensetzung und Menge, der von diesen Zellen produzierten Zytokine eine fundamentale Rolle für die Entwicklung und den Schweregrad der verschiedenen Asthma- Typen. Hierfür verwenden wir verschiedene Wege und Regime der Sensibilisierung. Diese Sensibilisierungen erfolgten über das Peritoneum, die Haut oder die Lunge, gefolgt von Provokationen über die Lunge. Außerdem verwendeten wir unterschiedliche Allergene (z.B.
Ovalbumin und Hausstaubmilbenextrakt). Neben dem Versuch, die Grundlagen der verschiedenen Modelle von akutem und chronischem Asthma zu verstehen, versuchen wir auch, einen tieferen Einblick in den Einfluss des genetischen Hintergrunds zu bekommen.
BALB/c-Mäuse werden immer noch häufig in der Tierforschung eingesetzt, obwohl C57BL/6- Mäuse der überwiegend verwendete Mausstamm sind. Da BALB/c-Mäuse bei einer Infektion mit Leishmania major eine Th2-Antwort zeigen, werden sie oft als "Th2-Responder"
bezeichnet, was bedeuten würde, dass sie einen schwereren Phänotyp in allergischen Atemwegsentzündungsmodell zeigen müssten. Unsere Ergebnisse zeigen allerdings, dass BALB/c-Mäuse eine konstant reduzierte Infiltration von Leukozyten in die Atemwege aufweisen. Dies ist hauptsächlich auf eine reduzierte Eosinophilie im Vergleich zu C57BL/6- Mäusen zurückzuführen.
Mastzellen (MCs) sind als Hauptakteure bei menschlichem Asthma bekannt. Es wird gezeigt, dass sie nach Allergenexposition wichtige Entzündungsmediatoren freisetzen.
Überraschenderweise ist ihre Rolle in Tiermodellen nach wie vor umstritten, was vor allem daran liegt, dass bisher keine spezifischen MC-defizienten Mausmodelle zur Verfügung standen, die keine MC-unabhängigen Effekte aufweisen. In dieser Arbeit verwenden wir Cpa3Cre/+ Mäuse mit funktionsfähigem Kit, die dennoch MC defizient sind. Diese Tiere zeigen einen ausgeprägten Mangel an MCs, sind aber (bis auf eine Reduktion der Basophilen) nicht von anderweitigen Effekten betroffen. Auch hier nutzten wir die oben genannten Modelle, um die Kontroversen um die Rolle der MCs in murinen Asthmamodellen aufzulösen. Wir können zeigen, dass das Fehlen von MCs in keinem der getesteten Modelle einen Einfluss auf die Schwere der allergischen Atemwegsentzündung hatte.
Die zweite Art von Immunantwort, die wir in dieser Arbeit umfassender charakterisieren, ist die Immunantwort bei Wundheilung. Das Zusammenwirken verschiedener lymphoider und myeloischer Zellpopulationen ist notwendig, um den Wirt vor dem Eindringen potenzieller Pathogene in die Wunde zu schützen und die Epithelzellen zu unterstützen, die Läsion zu schließen. Neben einer reinen Charakterisierung der infiltrierenden myeloischen Zellen versuchen wir auch Informationen über ihre Kinetik während des Heilungsprozesses erhalten.
Diese Analysen wurden in gesunden und heilungsgestörten diabetischen Mäusen durchgeführt. In frühen Wunden zeigen diabetische Mäuse reduzierte Zahlen und Prozentsätze von Makrophagen, aus Monozyten differenzierte dendritische Zellen (DCs).
Langerhans-Zellen, konventionelle DCs wie auch Eosinophile waren während des gesamten Heilungsprozesses in diesen Mäusen im Vergleich zu ihren gesunden Gegenstücken reduziert.
In der späten Phase der Wundheilung hingegen zeigen die heilungsgestörten diabetischen Mäuse einen deutlich höheren Anteil an Immunzellen. Dies könnte auf die Persistenz von Neutrophilen, Makrophagen und Monozyten zurückzuführen werden. Die konzertierten Aktionen von Immunzellen während der Wundheilung zu analysieren, insbesondere im Zusammenhang mit einer Wundheilungsstörung, kann helfen, chronischen Wunden zu verstehen. Dies bietet neue Möglichkeiten der Wundbehandlung.
Insgesamt zeigen die in dieser Dissertation vorgestellten Arbeiten die Zusammensetzung und Anzahl der Immunzellen die im Kontext der allergischen Atemwegsentzündung die Atemwege sowie das Lungengewebe infiltrieren. Des Weiteren, trägt diese Dissertation zu einer
9
sorgfältigeren Beschreibung der Infiltration sowie die Kinetik der myeloischen Zellen während der verschiedenen Stadien der Wundheilung bei.
11
3 Introduction
3.1 Immune System
The immune system protects the body of an organism throughout its life against potential invaders. Most living organisms face a constant threat, which depending on their size, can range from viruses to worms. While bacteria only have limited possibilities to protect themselves from potential invaders the immune system of animals is far more complex and has an array of offensive, defensive and regenerative mechanisms to protect the body.
The immune system of higher mammals such as humans and mice has evolved into two distinct interacting networks of cells and molecules, the innate and adaptive immune system.
These two systems are distinct in their response to threats, with the innate immune system characterized by its rapid and nonspecific response. In contrast to this, the adaptive immune system reacts in a decidedly slower fashion to an infiltrating pathogen, but counterbalances this delayed response with absolute precision.
The above mentioned innate immune system is the first line of defense against pathogens and will be discussed in Section 3.2. This includes which includes physical barriers (e.g. skin, the gastrointestinal tract, the respiratory tract, the nasopharynx and body hair), defense mechanisms (e.g. secretions, mucous, bile, gastric acid, saliva, tears and sweat) and the cellular response, which relies mainly on phagocytosis.
This first line of defense is followed by a second response that forms a highly specific attack against the invading pathogen. This response is orchestrated by the so-called adaptive immunity, which will be discussed in 3.3. Adaptive immunity, in contrast to the innate immunity, has the unique ability known as “memory formation”. In the event of a subsequent exposure by the same organism, this ability allows the adaptive immune system to mount a faster and more vigorous response, or in some cases even prevent a re-infection.
3.2 Innate Immunity
As previously alluded to an organism’s first line of defense is usually the adaptive immune system, or more specifically its physical barriers. Attacking microbes must first cross a surface barrier, such as a skin or mucus membrane to establish an infection. Epithelial cells attempt to prevent the attachment of infiltrating bacteria by producing anti-microbial peptides (AMPs), broad spectrum antibiotics that have been shown to kill Gram positive and Gram-
negative bacteria 1,2, envelope viruses and fungi. AMPs are very diverse, with over 880 possible peptides that can be grouped into families. Even though the primary mechanism of microbial killing by AMPs – formation of transmembrane pores that eventually lead to the lysis of the microbial cell – is well understood, this might not their only killing pathway 2. If a pathogen manages to breach the epithelial barrier an inflammatory response is mounted
3. The major players in the adaptive immune response are the complement system and innate leukocytes. The complement system is a biochemical cascade of proteins which are mostly synthesized by hepatocytes in the liver. These proteins perform two main actions, the first of which is to trigger the recruitment of inflammatory cells. The second action is to coat the surface of pathogens and thus “tag” it for destruction by immune cells. This coating can also cause the formation of holes in the plasma membrane, resulting in the death of the pathogen.
The complement system is, from an evolutionary standpoint, a very old pathway, and elements of it can be found in birds, fish, plants and even some species of invertebrates 4. The cells of the innate immune system in combination with the complement system can respond to infiltrating pathogens immediately. Unlike the antigen-specific receptors of the adaptive immune system, the receptors of the innate immune system are genetically predetermined and do not undergo genetic rearrangement to widen their specificity. These pattern-recognition-receptors (PRRs) recognize highly conserved molecular structures expressed by various microbes. These structures, known as pathogen associated molecular patterns (PAMPs) and danger associated patterns (DAMPs), are usually crucial for survival or microbial virulence and therefore are less prone to mutation than most other pathogen associated molecular structures 5-7. While PAMPs are derived from microbes, DAMPs are host-derived cell components that, when miss-localized or altered due to tissue injury, bind to PRRs. Examples of DAMPs include IL-33, HMGB-1 and S100 proteins, which are often released during inflammatory types of cell death, such as necroptosis. PRRs can be subdivided into two major groups defined by their localization in the cell. The first group are membrane- bound, and can appear on the cell’s surface, where they survey the extracellular environment.
The other location in which they can be found are the endosomes where that play a similar role, surveying endosomal compartments for the presence of microbial products. This membrane bound group includes the families of Toll-like receptors (TLRs) and the C-type lectin receptors (CLRs). The second group of PRRs are located in the cytosol, where they detect intracellular pathogens. This group includes the families of the nucleotide-binding
13
domain (NOD-like) receptors (NLRs) and the AIM-2-like receptors (ALRs) as well as the RIG-I- like receptors (RLRs) 4,8.
Binding of a PAMP or DAMP to a PRR leads to transcriptional changes. A large range of changes are possible including the production and release of pro-inflammatory cytokines (such as interferons (IFN)), phagocytosis, proteolytic cleavage and even cell death.
All five families of PRR that are highly expressed, are mainly (but not exclusively) found on myeloid cells such as macrophages (Section 3.2.4), dendritic cells (DCs) (Section 3.2.1), neutrophils (Section 3.2.8), eosinophils (Section 3.2.2), natural killer (NK) cells (Section 3.2.7) and mast cells (MCs) (Section 3.2.5). Neutrophils, basophils, and eosinophils are all commonly referred to as polymorphonuclear leukocytes (PMNs)
“Danger” signals trigger the adaptive immune system to be activated, however the system can also be activated due to so called “missing-self” signals. In humans and mice, all nucleated cells express major histocompatibility complex class I (MHC I), which engages inhibitory receptors on NK cells. If the balance of inhibitory and stimulatory signals diviates NK cells are activated and subsequently kill the respective host cell 9.
While macrophages, NK cells, innate lymphoid cells (ILCs) are tissue resident and patrol the sites of possible pathogen entry, other cells of the innate immune system, such as monocytes and neutrophils, are recruited in vast numbers upon inflammation 10.
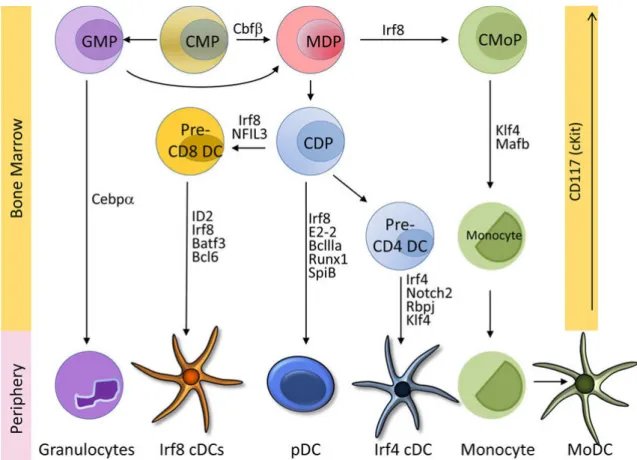
3.2.1 Dendritic cells
Dendritic cells (DCs) are professional antigen presenting cells (APCs), and as such their main function is the processing and presentation of foreign antigens on the cell surface to naïve T cells. In performing this function DCs connect the innate and adaptive immune systems, acting as a messenger between them. DCs can be found in all tissues that have contact with the external environment, such as skin, lungs, stomach as well as intestines. DCs can be activated by the binding of PAMPs or DAMPs to their PRRs 11. Once activated they migrate to the lymph nodes where they interact with naïve T cells to initiate the adaptive immune response. For their activation and subset differentiation, naïve T cells need three distinct signals, all of which can be provided by DCs. Signal 1 is the presentation of an antigen in the context of a major histocompatibility complex (MHC). This is recognized by the antigen- specific T cell receptors (TCR) on naïve T cells. Signal 2 is given by co-stimulatory molecules such as CD80 and CD86 on the DC, which bind to CD28 that is expressed on T cells. The binding
of costimulatory molecules stabilizes the so-called “immunological synapse” and generates signals that promote activation and survival. The last signal needed in order to activate a T cell is given by cytokines binding of cytokines (produced by APCs), binding to their receptors on the T cell. This final signal is especially important as it acts to polarize the T cell towards a different effector subset (Section 3.3.1.1) 12. These functions of DCs have made them a focus of research into using them for vaccines, tumors and treatment of autoimmune diseases.
Today DCs are subdivided into three major subsets, conventional DCs (cDCs), plasmacytoid DCs (pDCs) and monocyte derived DCs (moDCs) all of which will be described in the following sections.
Figure 1 Stages and transcription factors for DC development (Adapted from Murphy et al, 2016)13
3.2.1.1 Conventional DCs
cDCs are the most common DC subset. They can be found in nonlymphoid tissues and in the spleen marginal zone and they have the extraordinary ability to sense, capture and process foreign antigens for presentation to T lymphocytes. cDCs develop from the common dendritic progenitor (CDP) and express high amounts of MHCII and the integrin CD11c in most tissues.
15
In steady state and in the inflammation - antigen loaded cDCs migrate to the nearest lymph node (LN). With their ability to provide (or withhold) the three signals needed for T cell activation cDCs can trigger an immune response to any foreign antigen alternatively, and just as importantly, enforce tolerance to self-antigens 14. cDCs can be further subdivided into two branches.
cDC1s
cDC1s also known as Irf8+ cDCs or CD103+CD11b− DCs or XCR1+ DCs populate most connective tissues. These calls are also enriched in the Peyer’s patches of the intestine, where they express CD8 on the cell surface. While cDC1s do not express macrophage markers such as CD11b, CD115, CD172a and F4/80, they express higher levels (as compared to cDC2s) of Flt3, and proliferate in the presence of Flt3 ligand (Flt3L) 14. cDC1s have also been shown to be depended on transcription factors IRF8 and Batf3 13,15. The production of interleukin-12 (IL- 12) by this subset has shown to be important in the body’s defense against intracellular pathogens 13. While cDC1s have an immune regulatory function and help to maintain tolerance in steady state, in an inflammatory context they promote CD8+ T cell responses.
Although cDC2s have the ability to cross-present some forms of antigens (i.e. presentation of exogenous antigens on MHC I molecules to CD8+ T cells), cDC1s are specialized to do so 16. Due to these properties priming cDC1s with vaccine antigens has been proven to induce cytotoxic T cells and antibody 15.
cDC2s
cDC2s also known as Sirpa+ (CD172a) CD11b+ DCs or Irf4+ cDCs can be considered a more heterogeneous subset than their cDC1 counterpart. This set consists of Notch2-dependent Irf4+ cDCs and Klf4-dependent Irf4+ cDCs 13. These cDCs are dependent on the transcription factors RelB and IRF4 and rely on them for their development. Unlike cDC1s they are specialized for MHC II-restricted antigen presentation. They are also thought to primarily induce Th2 and Th17 responses 17.
3.2.1.2 Plasmacytoid DCs
pDCs refers to a small subset of DCs which can mainly be found in the blood and lymphoid tissue. Unlike cDCs, pDCs only express only low levels of MHCII and CD11c. When activated through the binding of nucleic acids to the TLR 7 and 9 present on their surface, pDCs produce vast amounts of type I IFN. Only after this has occurred do pDCs acquire the capacity to present antigens to T cells 14. A major notion is that pDCs can induce tolerance by expressing indoleamine 2,3-dioxygenase (IDO), the inducible costimulator ligand (ICOS-L) and the programmed death 1 ligand (PD-L1). This mediates regulatory T-cell (Treg) development as well as suppression of self- and alloreactive cells 18.
3.2.1.3 Monocyte-derived DCs
Unlike conventional and plasmacytoid DCs, “inflammatory DCs” or rather moDCs do not develop from the CDP (see Figure 1). Monocytes recruited to the site of inflammation can upregulate the DC markers MHCII and CD11c and acquire some functions characteristic of DCs, especially antigen presentation and to some extent migration to the LNs 17.
3.2.2 Eosinophils
Eosinophils are best known for their ability to fight multicellular parasites. They were names after their tendency to stain with acidic dyes, which is due to the vast number of highly basic proteins that are processed in their granules 19. Eosinophils develop from the common myeloid progenitor (CMP) via the myeloblast in the bone marrow; the most important transcription factors involved in their differentiation are GATA3 and C/EBP 20. The cytokine IL- 5 (mostly produced by T lymphocytes and ILCs is important for this development but can also be considered a key mediator of eosinophil functions 21. Mature cells circulate in the blood until they reach a site of inflammation or helminth infection. Eosinophils follow a trail of chemokines, for example eotaxin-1 (CCL11), eotaxin-2 (CCL24) or CCL5 (RANTES). At the site of inflammation eosinophils get activated and undergo cytolysis. These dying cell releases eosinophilic granule and extracellular DNA traps 21. Activated eosinophils are highly effective in killing multicellular parasites, however tin doing so can cause extensive tissue damage. In healthy individuals, eosinophils make up 1-3% of all white blood cells. This number increases
17
dramatically during helminth infections or asthma, if the number of eosinophils exceeds 500 eosinophils per microliter of blood, it is considered eosinophilia.
3.2.3 Innate lymphoid cells
Innate lymphoid cells are the most recently discovered innate immune cell type. As their name suggests they develop from common lymphoid progenitors (CLPs). After leaving the bone marrow they seed various lymphoid and non-lymphoid tissues, however they are most abundant at mucosal surfaces. The major function of ILCs is the secretion of signaling molecules. Furthermore, they play a key role in mucosal immunity and tissue homeostasis.
Unlike B and T lymphocytes, ILCs lack RAG expression and as such, no rearrangement of the antigen receptors can be found in them. In 2013 ILCs were divided into three groups according to their developmental pathways, phenotype, and the signaling molecules produced. Group 1 ILCs include ILC1s as well as NK cells. Group 2 ILCs only contain ILC2s, while group 3 ILCs contain NCR+ ILC3s, NCR– ILC3s and LTi cells 22. The roles of the different groups of ILCs are similar to those performed by the different T helper subsets. As such group 1 ILC share functions with Th1 cells, group 2 ILCs with Th2 cells and group 3 ILCs with Th17 cell. The dysregulation of ILCs has been linked to asthma and autoimmune disease 23.
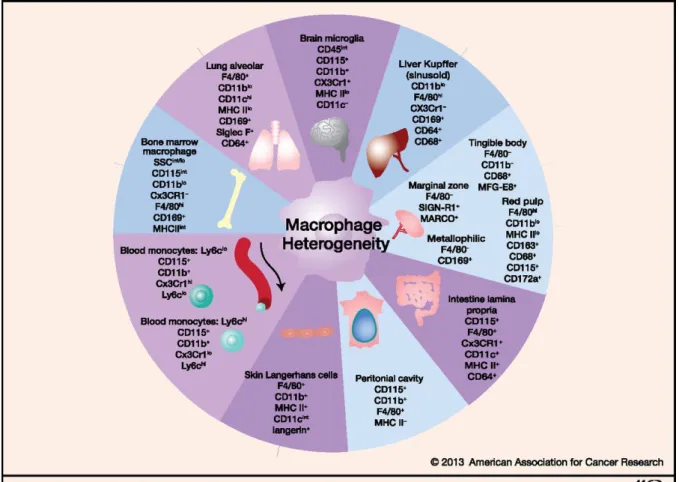
3.2.4 Macrophages
Macrophages are names after their function as large phagocytotic cells (μακρός (makrós) = large, φαγεῖν (phagein) = to eat). Specialized macrophages can be found in virtually all tissues (Figure 2), including them Kupffer cells in the liver, alveolar macrophages in the lung and microglia in the brain. The different tissues are seeded with macrophages during different stages of the development, fetal monocytes can replace primitive macrophages in tissue macrophage development 24. The main task of macrophages is to patrol the tissue and remove potential pathogens as well as cell debris. During steady state macrophages numbers are maintained through local proliferation.
Figure 2 Macrophage heterogeneity (Adapted from Lavin et al, 2013)25
Besides their phagocytotic ability, these cells also play an important role as APCs releasing both pro- and anti-inflammatory agents. Depending on the pathway activated, two subtypes of macrophages can be defined. Simplified, M1 macrophages (activated by Th1 cells) encourage inflammation, while M2 macrophages (activated by Th2 cells) promote tissue repair 26. This difference in activation also be seen in their metabolism 27.
3.2.5 Mast cells
MCs were first described by Paul Ehrlich in 1877 and are easily recognized by their unique staining characteristics and large granules. Ehrlich believed the function of MCs was to nourish the surrounding tissue. This lead to their name as the German word “Mast” translates to the “fattening” of animals 28. Nowadays MCs are known to be granulocytes and part of the adaptive immune system located in the connective tissue. Even though MCs are best known for their role in allergy and anaphylaxis, they are thought to also play important roles in wound healing, angiogenesis, immune tolerance, defense against pathogens, and vascular
19
permeability in brain tumors 29,30. In the airway of healthy humans, MCs can only be found adjacent to blood vessels in the lamina propria of the airway mucosa. In asthma patients MCs migrate to the airway smooth muscle 31 as well as to mucus glands 32. Even though MCs and basophils are similar in both function and appearance, it has been shown that the two cells types develop from different hematopoietic lineages 33.
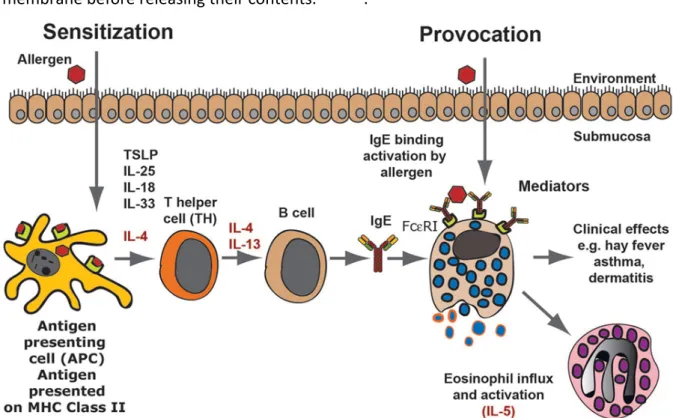
Due to their lack of antigen specific receptors MCs are part of the innate immune system, however their expression of the high-affinity FceR1, still allows them to react in an antigen specific manner. FceR1 is a tetramer consisting of one alpha (a), one beta (b) and two gamma (g) chains. Immunoglobulin (Ig) E can bind to the extracellular portion of the a chain 34-36. When an allergen binds to membrane bound IgE, FcεR1 molecules cross-link and signal is transduced through the immunoreceptor tyrosine-based activation motif (ITAM) on the β and γ chains 37. At the end of this signaling cascade the tyrosine-protein kinase Syk is activated and phosphorylate multiple target proteins, resulting in an amplification of the signal 38. This pathway leads to degranulation and release of the aforementioned large granules (Figure 3).
These granules, which are rich in histamine, heparin and leukotrienes, fuse with the plasma membrane before releasing their contents. 35,39-41.
Figure 3 An allergic response (Adapted from Hellman et al, 2017)42
MCs are also an important source of cytokines, chemokines, and growth factors 43-45. However, due to their capacity to degranulate, mast cell can impact the body in a negative manner by causing or exacerbating a variety of disorders. A prime example of this are allergic diseases; which occur mediated through IgE signaling 46. Thus, re-exposure to the original allergen will lead to cross-linking of membrane bound IgE and subsequent degranulation. MCs play an important role in the pathophysiology of asthma, eczema, allergic rhinitis, allergic conjunctivitis and a large range of disorders that present with an itch. Many symptoms of these diseases can be alleviated or treated using antihistamine drugs.
The most severe form of allergic reaction is anaphylaxis, which leads to body-wide degranulation of MCs. The enormous amount of histamines realized into the body during anaphylaxis leads to vasodilation and thus to a drop in blood pressure, which may result in a life-threatening shock 47.
Even though mast cells are best known (and best understood) in the context of allergic disease and anaphylaxis research done in the last fifteen years has shown mast cells also play an important role in the resistance to snake and honeybee venom 48.
3.2.5.1 Mouse models of mast cell deficiency
Studies on the function of MCs in mice, depend on the use of animals bearing mutations that either prevent the mast cells from developing, or alternatively render them nonfunctional.
One of these mutations is the pro-oncogene c-kit. The KIT receptor, also known as CD117, binds the main MC growth and survival factor stem cell factor (SCF). Mice lacking this gene are deficient in mast cells but also suffer from a wide range of additional confounding effects, that are not an inherent consequence of the absence of mast cells. There are currently two stains in common usage that possess mutations in this gene, the WBB6F1-KitW/W-v and C57BL/6-KitW-sh/W-sh mice. Both stains show a profound lack of mast cells, but also exhibit aberrations, both in- and outside of the immune system 49,50. For this reason, models of MC- deficiency that preserve KIT signaling are desirable. Two stains that retain functional KIT are Mcpt5-Cre; R-DTA and Cpa3Cre/+ - ‘Cre-Master’ mice. Both these stains lack MCs but are mostly unaffected by MC-independent aberrations. Cpa3Cre/+ mice, which were created by inserting Cre-recombinase into the locus encoding mast cell carboxypeptidase A3, have been shown to completely lack MCs (due to Cre-mediated toxicity) 50. While these mice also exhibit a partial reduction in the numbers of basophils, all other cells of the immune system were shown to
21
be unaltered 50. This makes Cpa3Cre/+ mice an ideal model to study the impact of MC and MC deficiency on the pathogenesis of allergic airway inflammation (AAI).
3.2.6 Monocytes
Monocytes are large leukocytes mostly found in the blood. Upon inflammation these cells can differentiate further into macrophages or monocyte derived DCs. In steady state tissue resident macrophages will self-renew. However, in the context of inflammation they will instead rely on the additional numbers from monocytes derived cells. In mice two subsets of monocytes can be discerned. Inflammatory monocytes and resident monocytes, which differ in their expression of the marker Ly-6C 51,52. The main functions of monocytes and their progeny are phagocytosis, antigen presentation and cytokine production. Cytokines produced by monocytes are among others TNF, IL-1 and IL-12.
3.2.7 Natural killer cells
NK cells are cytotoxic lymphocytes, they provide a rapid response to virus infections (in the order of three days after infection) and tumor formations. NK cells have the unique ability to recognize and kill stressed cells (without antibody or MHC recognition). This ability is essential to kill cells that do not express the “self”-marker MHC class I. NK cells develop in the bone marrow from the common lymphoid progenitor (like the B and T lymphocytes in the adaptive immune system), but since they are part of the innate immune system they belong to the group of ILCs 53. Besides their function as effector cells, NK cells also have an important task as inhibitory cells, and as such they induce self-tolerance. Further roles of NK cells are still under investigation especially in the context of cancer therapy 54,55.
3.2.8 Neutrophils
Neutrophils are the most abundant leukocyte; they make up 40-70% of all white blood cells in humans. Neutrophils develop in the same way as other granulocytes, developing from CMP in the bone marrow. After leaving the bone marrow they are relatively short-lived 56 (their live expectance is thought to be around five hours) leading to the human body producing approximately 1011 neutrophils per day. Together with eosinophils and basophils they are part of the family of polymorphonuclear cells 57,58, and as such possess a the nucleus that is
usually segmented into 2-5 lopes. At the start of an inflammatory response, due to bacterial infection or environmental exposure, neutrophils are one of the first responders. Neutrophils leave the blood stream following chemical signals such as IL-8, C5a, fMLP and arrive at the site of injury within minutes of trauma occuring. This neutrophil swarming can be considered a hallmark of acute inflammation. At the site of inflammation neutrophils undergo NETosis, a process in which cells release granule proteins and chromatin to form an extracellular fibril matrix 59.
3.3 Adaptive Immunity
The adaptive immune system, in contrast to the innate immune system, is able to mount a highly specific attack against an invading pathogen and to form memory.
The unique ability of forming a highly specific response is due to random recombination and mutation of gene segments. This leads to a distinctive antigen receptor on each cell 4. While the innate receptors are expressed on a huge variety of cells (hematopoietic and non- hematopoietic cells alike), receptors derived from gene recombination can only be found in B and T cells. The possible diversity of the TCRs and B cell receptors (BCRs) that can be reached using this mechanism is estimated to be between 1012 and 1015. This extremely high diversity allows the adaptive immune system to have a distinct and highly specific receptor for a particular antigen, but also leads to an exceptionally low frequency of any given receptor. Due to the low frequency of cells carrying any given receptor there is the need for extensive cell expansion upon an encounter with a targeted antigen. The so-called clonal selection and expansion which performs this function is a cornerstone of the adaptive immune system 60 and is typically able to produce the required numbers of effector cells in five to seven days.
As mentioned previously the adaptive immune system is capable of generation and maintenance of effector derived memory cells that can be activated upon re-infection. This secondary immune response is faster and stronger than the primary response. This secondary response is the basis of vaccination.
3.3.1 T cells
T cell can be subdivided into two major populations the ab and gd T cells. This classification depends on if they express the a and b chains or the g and d chains in their TCR complex
23
(further discussed in Sections 3.3.1.1, 3.3.1.2 and 3.3.1.3). In humans 95% of the T cell population is made up of ab T cells. Due to their dominance for the remainder of this thesis, unless otherwise stated, we will simply refer to ab T cells as T cells.
All T cells develop from hematopoietic stem cells (HSC) in the bone marrow. These HSCs differentiate into multipotent progenitors (MPPs) and further into CLPs which give rise to T, B and NK cells 61. CLPs migrate to the thymus where they become T cells. This first stage of development, the so called “early thymic progenitors” (ETPs), can also be referred to as the double negative stage, due to their lack of CD4 or CD8 expression in the cell surface. Next CD4 and CD8 are both upregulated creating “double-positive” cells. Once this has occurred the thymocytes undergo positive selection which removes all cells that cannot bind to the MHC.
A thymocyte that interacted with an MHC class I molecule will down-regulate CD4 and become a CD8+ T cell, while a thymocyte that interacted with an MHC class II molecule will instead down-regulate CD8 an become a CD4+ T cell. This positive selection is followed by what is known as “negative selection”, whose main major purpose is to remove potentially autoreactive T cells. The process accomplished this by giving an apoptotic signal to all cells that interact too strongly with a self-antigen. Only 2% of all thymocytes leave the thymus and migrate to the periphery to fulfill their function as immunocompetent T cells.
3.3.1.1 CD4+ T cells
CD4+ T cells are generally considered the key orchestraters of the adaptive immune response.
Due to this fundamental role in immunity, these cells are also called T-helper cells (Th). CD4+ T cells are capable of steering the immune response in very distinct directions. The direction taken depends on a range of factors such as the strength of TCR binding, costimulatory molecule binding as well as the cytokine milieu.
This, together with antigen-specific receptor binding, allows the immune response to be purpose-built for any given pathogen. CD4+ T cells can be further subdivided into two major groups, effector T cells that promote inflammation by cytokine secretion and support B cell mediated antibody response and regulatory T cells (Treg). These Tregs limit the immune response by secreting anti-inflammatory cytokines to prevent excessive inflammation and the consequent host damage.
CD4+ T effector cells
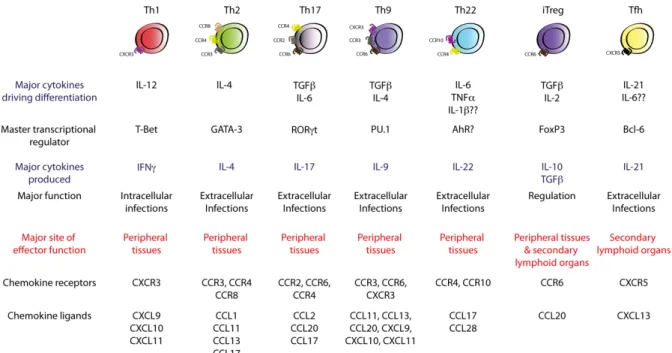
As mentioned previously CD4+ T cells have the ability to steer the immune response in distinct directions depending on the nature of the threat. Currently there are seven widely documented Th subsets. These are Th1, Th2, Th17, Th9, Th22, inducible Treg (iTreg) and T follicular helper (Tfh). Each linage can be defined by the signature transcription factors, the major cytokines driving the differentiation and the cytokines produced (Figure 4). Th1, Th2, Th17 and Tfh cells will be described in more detail below. Although the differentiated states are relatively stable, some degree of plasticity has been shown and the true number of Th subsets is a matter that remains open for debate 62.
Figure 4 Currently known Th cell subsets (Adapted from Kara et al, 2014)63
Th1 cells
Th1 cells are essential for the response against intracellular pathogens such as viruses and intracellular bacteria. Their major cytokine of differentiating cytokine is IL-12, which binds to the interleukin-12 receptor β2-chain (IL-12Rβ2) on naïve T cells. This eventually leads to IFNg expression. IL-12 and IFNg signaling is performed through signaling through transducer and activator of transcription 1 (STAT1) and STAT4 to induce T-box transcription factor TBX21.
TBX21 is more commonly referred to as Tbet and is the master transcription factor of Th1 cells. Tbet expression further increases IFNg expression as well as suppressing IL-4 expression.
25
Besides IFNg, the cytokine profile of Th1 cells includes also IL-2 and tumor necrosis factor (TNF). These cytokines lead to increased antigen presentation and killing of intracellular bacteria in macrophages and PMNs. In the adaptive immune system Th1 cytokines promote the development of cytotoxic lymphocytes, which are capable of killing cells that have virus infections or are tumorous.
Aberrant Th1 response can lead to a severe disorders such as multiple sclerosis (MS) and type- 1 diabetes as well as rheumatoid arthritis64.
Th2 cells
Th2 cells are important part of a host’s defense against large extracellular parasites, such as nematodes and helminths. The cytokine profile that defines Th2 cells is comprised of IL-4, IL- 5 and IL-13, in addition to this, Th2 cells have been shown to produce IL-6, IL-9 and IL-25. Th2 cytokines are also capable of recruiting and activating eosinophils and basophils. Further functions of theirs include the polarization of macrophages to an alternatively activated or M2 phenotype, promoting mast cell hyperplasia, mucus production, goblet cell metaplasia and airway hyper-responsiveness (AHR). The latter is particularly relevant in the context of allergic immune responses, such as asthma, which will be discussed in Section 3.4.
Besides being essential for B cell class switching to IgE, IL-4 is can induce Th2 cell differentiation. This IL-4 signaling is thought to upregulate GATA3, the master transcription regulator of Th2, via the induction of STAT6. Interestingly it has been shown that the Th2 response is not exclusive to IL-4 and can also be induced by other cytokines, such as Thymic stromal lymphopoietin (TSLP), IL-25 and IL-33. In a similar manner to IFNg blocking IL-4 induction, IL-4 can in turn suppress Th1 and Th17 responses. However, GATA3 expression and STAT5 signaling hast been shown to be essential for Th2 differentiation 65.
Over or misdirected activation of Th2 responses are common allergic responses. Symptoms can range in their severity from redness and itching, all the way up to life threatening symptoms like shortness of breath and a rapid drop in blood pressure, as occurs during an anaphylactic shock 66.
Th17 cells
Th17 cells are instrumental in a host’s defense against extracellular bacteria and fungi. The major cytokines involved in a Th17 response are IL-17A, IL-17F and IL-22, but TNF, granulocyte-macrophage colony-stimulating factor (GM-CSF), IL-17AF and IL-21 can also be produced 67. Th17 differentiation is driven by transforming growth factor beta (TGFb) and IL- 6. The major transcription factors involved in a Th17 response are STAT3 and RAR-related orphan receptor gamma (RORgt). In vivo a dependency on IL-1b and IL-23 have been shown.
The latter stabilizes RORgt, which allows an intact and extended Th17 response 68. Besides STAT3 and RORgt a number of additional transcription factors, such as AHR, BATF, IκBζ, IRF4, c-Maf and RORα are also involved in this response.
Two major functiosn of the Th17 response are the recruitment to and activation of neutrophils at the site of an infection and the inducing the production of antimicrobial- peptides by epithelial cells. Since the epithelia surfaces (e.g. skin) and mucosal tissues (e.g.
intestinal tract), are the primary access points for infiltrating extracellular bacteria and fungi, these sites are the major points of action for Th2 cells. Th17 differentiation here is inhibited by IFNg and IL-4 cells produced by Th1 and Th2 cells respectively 69. Nevertheless, as shown in our previous work, we see a predisposition of Th17 cells to upregulate Th2 cytokines upon challenge. This finding indicates a closer developmental connection between Th17 and Th2 cells than previously anticipated 62. Th17 cells are involved in a plethora of disorders which include but are not limited to severe asthma, psoriasis, rheumatoid arthritis, inflammatory bowel disease (IBD), MS and many more.
Tfh cells
The main function of T follicular helper cells is the secretion of IL-21 and assisting the consequential B cell-mediated antibody response. To differentiate into Tfh cells, naïve T cells require IL-6 in addition to this IL-21 and B cell interaction. The predominant transcription factor for the Tfh function is B-cell lymphoma 6 (Bcl6). Bcl6 is required to perform for changes in C-X-C chemokine receptor type 5 (CXCR5) and C-C chemokine receptor type 7 (CCR7) expression, without which the Tfh could not migrate to the B cell follicle. Another important task of Bcl6 is the repression of other master transcription factors such as RORgt, T-bet and GATA3 70. When stimulated with antigens Tfh cells migrate to the follicular region of the
27
secondary lymphoid organs (SLOs) such as the lymph nodes (LNs), spleen, Payer’s patches (PPs) mucosal tissues- the nasal associated lymphoid tissue (NALT), adenoids, and tonsils 71. At the edge of the B cell zone, Tfh cells interact with B cells to generate germinal center reactions and promote a long-lasting humoral response. Besides IL-21, Tfh also produce IL-4, IL-10 and IFNg to stimulate class-switching and germinal center formation of mature B cells.
Due to their role in B cell activation Tfh cells have been linked to a number of diseases, these include systemic lupus erythematosus and Sjogren’s syndrome as well as other disorders connected to an aberrant antibody response 72.
Additional T cell subsets
Besides the T helper subsets described in detail above, other less established T helper cells have been discovered. These are shown in Figure 4.
Th9 cells, which produce IL-9 have been proposed to be involved in the immune response against parasitic worms, and are similar to, but distinct from Th2 cells. Th9 differentiation can be induced by the cytokines IL-4 and TGFb. While Th9 cells do not produce typical Th2 cytokines such as IL-4, IL-5 and IL-13 they do express GATA3 73. PU.1 is generally considered to be their master transcription regulator. However, this expression of GATA3 has given cause to the debate as to if Th9 is truly a distinct linage, or should rather be considered a Th2 subset.
In a similar way to Th2 cells, Th9 cells have been implicated in causing inflammatory diseases of the intestinal tract as well as allergic inflammation.
Th22 cells, like Th9 cells, are a newly characterized linage of Th cells. They mainly secrete IL- 22 and control immunity at the mucosal barrier surface, in a similar, but distinct manner to Th17 cells. While Th22 cells do not produce linage defining cytokines such as IFNg, IL-4 or IL- 17 they can, under certain conditions, produce IL-13 and fibroblast growth factor (FGF). Since IL-22 takes effect on non-hematopoietic cells – mainly stromal and epithelial cells, it is important for cell survival, proliferation and synthesis of antimicrobials including S100, Reg3β, Reg3γ and defensins. With these functions, IL-22 participates in wound healing and in protection against invading microbes 74. Th22 differentiation requires TNFα and IL-6 (in vitro), the nature of the master transcriptional regulator is still under investigation. Similar to Th17 cells, Th22 cells have been shown to play a role in inflammatory diseases such as asthma, atopic dermatitis, psoriasis, rheumatoid arthritis and Crohn’s disease.
Regulatory T cells
Tregs, unlike the effector T cells described above, do not promote inflammation. Unlike what their name suggests, the major function of Tregs is not regulation of the immune response but rather suppression. This CD4+ T cells population can inhibit the function of T helper cells, for example cytokine expression and proliferation. Tregs can also suppress the function of APCs. Immunosuppression by Tregs is very important in maintaining the body’s peripheral tolerance to self-antigens, as well as inducing feto-maternal tolerance during pregnancy 75. The mechanism behind this immunosuppression are the cytokines IL-10, TGF-b and IL-35. The signature transcription factor for Tregs is FoxP3, which is essential for their function. Tregs can be further divided into two groups depending on their developmental. Natural Tregs (nTregs) develop in the thymus and gain their suppressive function upon leaving it. Induced Tregs (iTregs), on the other hand, leave the thymus as naïve CD4+ T cells and only become suppressive after being exposed to a specific cytokine environment 76. Since Tregs play a major role in balancing the immune response, defects in Treg function lead to autoimmune issues, lymphoproliferation, allergic dysregulation and ongoing lymphocytic infiltration in different organs 77.
3.3.1.2 CD8+ T cells
CD8+ T cells or cytotoxic T lymphocytes (CTLs) are like their CD4+ counterpart antigen specific.
Naïve CD8+ T cells are activated by dendritic cells in the lymph nodes, and after several rounds of expansion migrate to a site of inflammation. Activated CD8+ T cells are capable of killing virus-infected or tumor cells and have to means f doing so. The first of these is by secreting perforin, which created pores in the plasma membrane of a targeted cell. These pores allow granzyme to enter the cell and initiate apoptosis. The second pathway of cytotoxicity involves the binding of Fas ligand to Fas on a target cell, which will induce apoptosis in the Fas expressing cell. Besides their function as cytotoxic cells, CD8+ T cells produce IFNg and TNF;
both of which help in the defense against viral infection and anti-tumor response. Like Th1 T cells, which produce IFNg and TNF, CD8+ T cells express Tbet as one of their master transcriptional regulators 78. The other important transcription factor for CD8+ T cell differentiation is Eomesodermin (Eomes). Due to its proficiency in killing virus infected and
29
aberrant cells, CD8+ T cells are capable of causing major tissue damage. To prevent immunopathology and autoimmunity, CD8+ T cells that receive an excessive number of signals enter an “exhaustion” state. In this state they lose their effector functions, for example cell killing and cytokine production. While exhaustion reduces the chance of autoimmunity and immunopathology, CD8+ T cells also go into exhaustion when combating tumors 79. In addition to the CD8+ T cells subset describes above other less-well characterized subsets have been proposed. Of these the Tc17 cells are best understood and like their Th17 counterpart, mainly produce IL-17 while also producing some IL-21 and IL-22. RORγt and STAT3 show signs of being important for their differentiation. However, it is still under investigation as to whether Tc17 cells are a stable CD8+ T cells subset or rather a short-term state, as these cells readily produce IFNg as well as IL-17A 80.
3.3.1.3 gd T cells
Unlike CD4+ and CD8+ T cells, gd T cells express g and d chains in their TCR complex. Speaking from both an evolutionary and task wise perspective, they can be placed between the fast but “primitive” innate immune response and the slow targeted adaptive response. On the one hand, gd T cells rearrange their TCR genes to produce diversity and are capable of memory formation. On the other hand, they respond within hours in an antigen independent manner.
The frequency of gd T cells in the peripheral blood ranges from 0.5 - 10% in humans and mice, around 20% in sheep, goats and deer and up to 30% in pigs 81. In humans and mice gd T cells can be found at their highest abundance in the gut mucosa. How gd T cells get activated is still not fully understood. Their activation seems to be independent of the presentation of antigens on MHC molecules, even though some gd T cells recognize MHC Ib molecules 82. Their tasks during the immune response include being a “first line of defense” as well as bridging the innate and adaptive response. During their development gd T cells seed different tissues in specific waves. The first waves seed the skin with so called dendritic epithermal T cells (DETC), which while present in mouse skin, are absent in humans. Further waves seed the lungs, tongue, uterus, blood, spleen and LNs 83. Abnormalities in gd T cells function have been associated with many autoimmune diseases, such as idiopathic bowl disease, type 1 diabetes, rheumatoid arthritis, multiple sclerosis and psoriasis 84.
3.3.2 B cells
Besides T cells and NK cells, B cells are the last class of lymphocytes. Their primary task is the production of antibodies. In addition to this B cells are also capable of presenting antigens on MHC II and secrete cytokines 4. In mammals B cells develop from the CLP in the bone marrow;
in birds on the other hand, they develop in the bursa of Fabricius. As such the B in their name is owed to them first being discovered in the bursa of Fabricius. In the bone marrow B cells go through several stages of development, during which they also undergo V(D)J recombination. B cells undergo positive and negative selection, before leaving the bone marrow, which ultimately leads to clonal deletion, receptor editing, anergy, or ignorance.
Immature B cells leave the bone marrow and pass through the spleen (where they differentiate into follicular or marginal zone B cells) on their way to the secondary lymphoid organs (SLOs) 4. B cell activation begins with the binding of an antigen to the BCR. This can occur in a T cell-dependent or independent manner. After activation, B cells differentiate into short-lived effector cells or long-lived memory cells for persistent protection 85.
3.4 Asthma
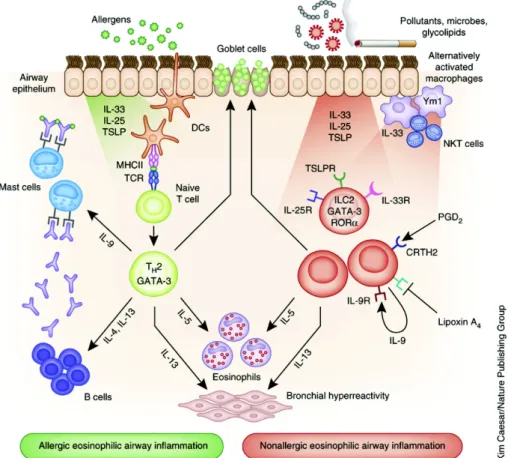
Asthma is a major non communicable disease of the airways of the lungs. Currently over 240 million people suffer from its effects 86, with 1 in 10 children and 1 in 12 adults in developed countries having been diagnosed with asthma 21. Hallmarks of asthma include reversible airflow obstruction, bronchial inflammation as well as airway hyperresponsiveness (AHR) 87. Patients suffer from a range of symptoms including shortness of breath, chest tightness or pain and wheezing when exhaling 88. While symptoms can be prevented by avoiding triggers or ameliorated by inhaling corticosteroid, there is no cure for asthma. The causes of asthma are still unknown; however it is widely agreed upon that both environmental (e.g. exposure to air pollution and allergens) and genetic factors play a role 89-92 (see Figure 1).
31
Figure 5 The immune response in asthma (Adapted from Lambrecht et al, 2015)21
For the last decades asthma was often considered a prototypical type 2 allergic response. This belief stemmed from the fact that Th2 cells and ILC2s were thought to be the predominant producers of the effector cytokines IL-4, IL-5 and IL-13 93-97. Even though Th2 cytokines have been shown to be instrumental in mediating several key features of asthma and mice lacking these cytokines have been shown to lack eosinophilia, IgE synthesis, baronial hyperresponsiveness and airway remodeling 94,96,97, this paradigm has shifted. The importance of Th1 cytokines has been shown by treating mice with IL-12 to induce the production of interferon-g (IFN-g) , which suppressed certain aspects of asthma in mice 98,99. Surprisingly, it is now estimated that up to 50% of clinical patients with persistent asthma do not possess a Type 2 response in their airways 100. Furthermore, when the airways of both patients with severe asthma and individuals that have died from sudden-onset asthma are examined neutrophil numbers typically exceeded eosinophils numbers 101-103.
In response to these findings, the immunological opinion about asthma has changed and various endotypes that present differently in the clinic and require different therapy have been established 100,104.
Along with these changes, the differentiation between eosinophilic asthma (EA) and non- eosinophilic asthma (NES) has become more and more relevant 105. While EA has been studied intensively, the mechanisms behind, and treatment of NES are less well understood 106,107. On the Th2 side, it has been shown that IL-4 is important for the generation of adaptive Th2 immunity and for eosinophil extravasation into the lung 108. IL- 13, on the other hand has been shown to induce mucus production from goblet cells 97. Surprisingly, eosinophils are not critical for the disease’s development, but rather exacerbate inflammation by promoting AHR and promote airway remodeling via TGF-β production in some asthma models 109.
The other major producer of Th2 cytokines are ILCs (most notably ILC2s). As part of the innate immune system, they can respond rapidly to epithelial-derived cytokines such as IL-25, TSLP and IL-33, which this can promote eosinophil recruitment and mucus production.
On the Th17 side, the role of IL-17A and IL-22 are not, as of now, fully understood.
Nevertheless, it seems that the expression of these cytokines is dependent on the phase of inflammation. IL-17A appears to function in a protective role during the acute allergic inflammation. Conversely the transfer of Th17 cells has been shown to enhance Th2-driven immunity 110. In the late stages of inflammation Th17 cells contribute to AHR and airway remodeling 111. IL-22 can promote allergic sensitization but has been found to eventually reduced the inflammatory response during the challenge phase of AAI models 112. Mice deficient in IL-22 have been found to suffer from higher AHR upon airway challenge 113. Further cytokines that play a crucial role in the development of asthma are IL-25, TSLP, GM- CSF and IL-33. These cytokines influence multiple key cell types, ultimately leading to the production of Th2-recruiting chemokines CCL17 and CCL22.
From this we conclude that the complexity of asthma and its various endotypes observed in human patients as well as in experimental asthma models highlights the need for further research of the molecular factors involved in the disease’s development.
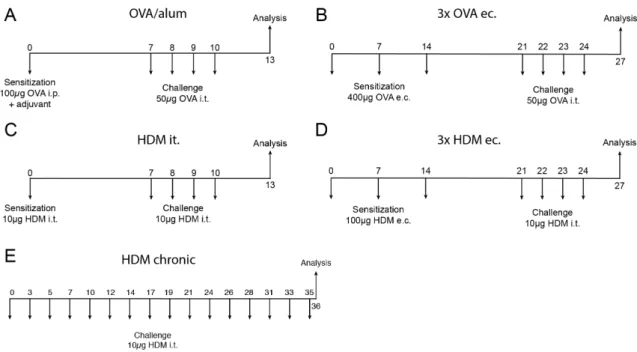
3.4.1 Murine models of allergic airway inflammation
Animal models of asthma are instrumental in improving our understanding of the disease.
Deeper insight into the endotypes of asthma in humans have resulted in the number and variety of murine asthma models growing. Each model comes with its own advantages and disadvantages, fitting to the range of key features and hallmarks of the human disease with varying levels of accuracy. The models use a variety of allergens and routes of immunization
33
as well as different sensitization regimes. Much of the early work in this area has been performed using one of the most common experimental models of asthma. This model is driven by sensitization with ovalbumin (OVA) and Alum and subsequent inhalation of OVA.
Today, models using house dust mite (HDM) extract are generally preferable, as HDM is considered a more physiological allergen. Even though many of these models have been described in detail, they are rarely compared to one another. One of the primary contributions of this thesis will be to rectify this through the comparison of several commonly used models of AAI.
35
4 Results
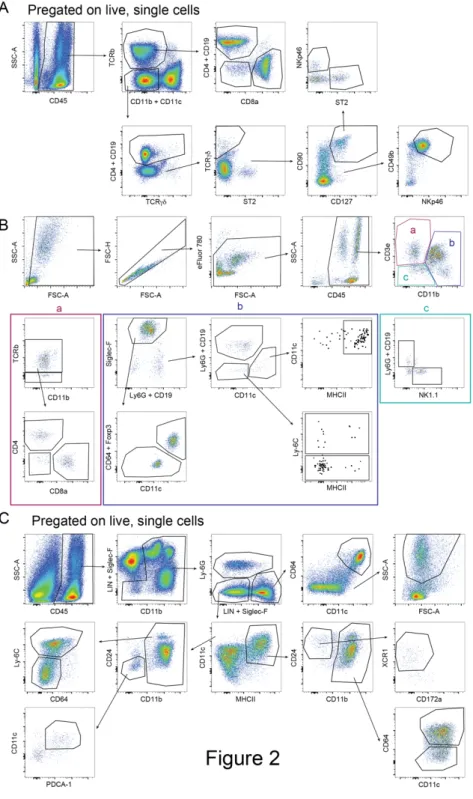
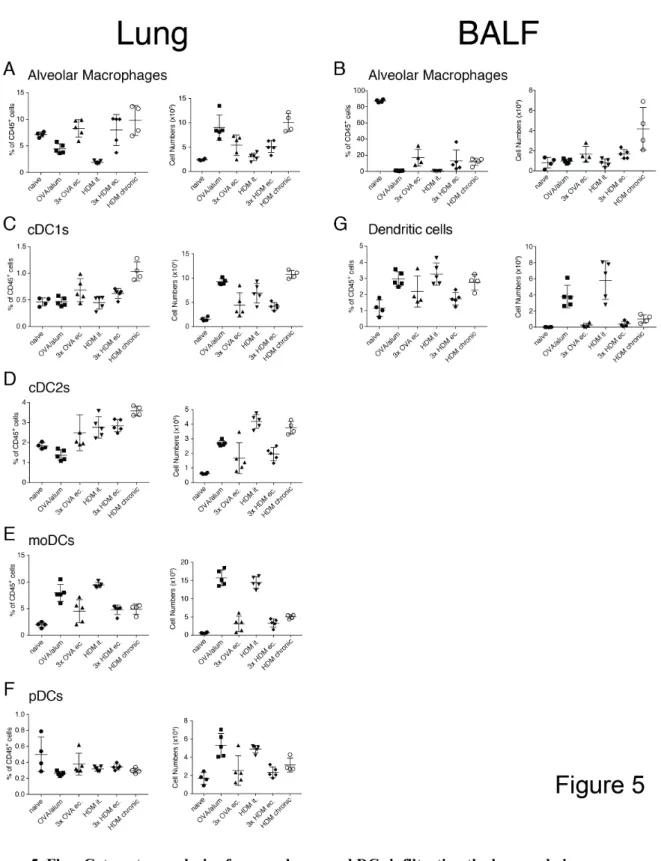
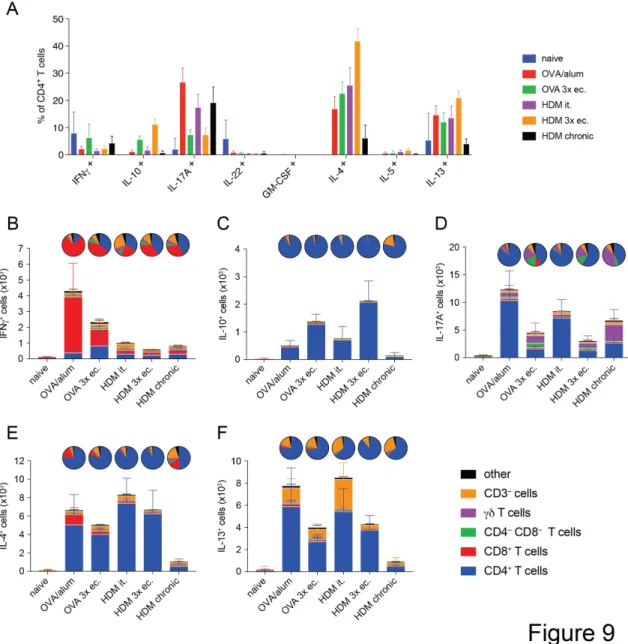
4.1 Comparison of airway inflammation and T cell responses in different acute and chronic mouse asthma models
Manuscript in preparation.
Comparison of airway inflammation and T cell effector responses in different acute and chronic mouse asthma models
Lea Pohlmeier1, Luigi Tortola1 and Manfred Kopf1
1 Institute of Molecular Health Sciences, ETH Zurich, Zurich, Switzerland.
Abstract
Over the last decades a multitude of different murine asthma models have been established with the goal of addressing the mechanisms of asthma. Each of these models manages to emulate features of the human disease and leads to a different composition in cells infiltrating into the lungs. Cytokines play a fundamental role in the development and severity of the different types of asthma. Furthermore, many aspects such as age, gender and microbiota can influence the severity and outcome of the model. This makes it arduous to compare models used in different labs and draw conclusions about the advantages and disadvantages of those models in a meta-analysis.
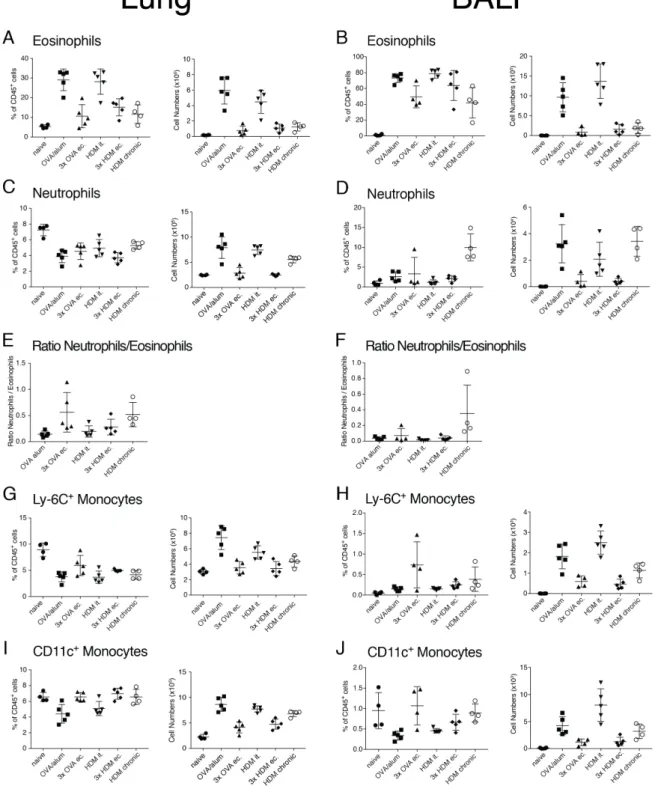
The objective of this study was to provide comprehensive analysis of the most common models of asthma and analyze the diversity in cytokine responses generated in these models.
We found that the number of infiltrating cells into the lung tissue and the airways differs greatly between the tested models. Also, the chronic model showed the most pronounced difference in cellular response compared to the acute models. Furthermore, it can be noted that not just the number of cytokine-producing cells but also the composition of cytokine producers changes between the tested models.
Introduction
Asthma is considered one of the major non communicable diseases worldwide with over 230 million people currently suffering from its effects 1. The pathological hallmarks include reversible airflow obstruction, bronchial inflammation as well as airway hyperresponsiveness (AHR) 2. Wheezing, coughing, shortness of breath, and chest tightness as well as pain 2 are considered the most common symptoms. Even though the causes of asthma are still unknown it is agreed upon that environmental as well as genetic factors are involved 3-6.
For the last 30 years, asthma was considered a prototypical type 2 allergic response with interleukin-5 (IL-5), IL-4 and IL-13, predominantly produced by Th2 cells and ILC2, mediating key features including pulmonary eosinophilia, IgE antibody responses, airway hyperresponsiveness, goblet cell hyperplasia and mucus production 7-11.
Mice lacking IL-5 are protected from lung eosinophilia but develop goblet cell hyperplasia. IL- 13-deficient mice are protected from airway hyperresponsiveness and goblet cell hyperplasia.
Notably, mice lacking IL-4R, which is required for both IL-4 and IL-13-signaling, are completely protected from all asthma features including eosinophilia due to impaired development of IL-5 producing T cells 8,10,11. Inducing the production of interferon-g (IFN-g) by treating mice with IL-12 even suppressed certain aspects of asthma in mice 12,13.
Even though CD4+ T cells producing IL-5 are commonly found in lung lavage and blood of asthma patients 14,15 it is now estimated that up to 50% of clinical patients with persistent asthma do not show type 2 immunity in their airways 16. Surprisingly, in the airways of patients with severe asthma as well as those who died from sudden-onset asthma, neutrophil numbers exceeded eosinophils numbers 17-19.
In the last decades the notion that asthma is not a purely Th2-dependent disease grew more and more. Various endotypes that present differently in the clinic and require different therapy have been established 16,20.