Research Collection
Doctoral Thesis
The role of keratinocyte-expressed podoplanin in skin carcinogenesis and wound healing
Author(s):
Sesartić, Marko Publication Date:
2020
Permanent Link:
https://doi.org/10.3929/ethz-b-000454790
Rights / License:
In Copyright - Non-Commercial Use Permitted
This page was generated automatically upon download from the ETH Zurich Research Collection. For more information please consult the Terms of use.
DISS. ETH NO. 26755
The role of keratinocyte-expressed podoplanin in skin carcinogenesis and wound healing
A thesis submitted to attain the degree of Doctor of Sciences of ETH Zurich
(Dr. sc. ETH Zurich)
presented by Marko Sesartić
MSc. ETH in Biology, ETH Zürich
born on May 14, 1991 citizen of Croatia
accepted on the recommendation of Prof. Dr. Michael Detmar, examiner Prof. Dr. Sabine Werner, co-examiner
2020
Per aspera ad astra.
Table of Contents
1. Summary/Zusammenfassung 1
1.1. Summary 1
1.2. Zusammenfassung 3
2. Introduction 5
2.1. The lymphatic vasculature 5
2.1.1. Anatomy of the lymphatic vasculature 5 2.1.2. Development of the lymphatic vasculature 8
2.1.3. Lymph node development 11
2.1.4. Main lymphatic growth factors and signaling pathways 12 2.1.5. Lymphatic vessels in inflammation 14
2.1.6. Lymphatic vessels in cancer 17
2.1.7. Lymphatic vessels in wound healing 18
2.2. Podoplanin 19
2.2.1. Podoplanin structure 19
2.2.2. Podoplanin in embryonic development and differentiation 22 2.2.3. Transcriptional control of podoplanin expression 24
2.2.4. Podoplanin in pathology 25
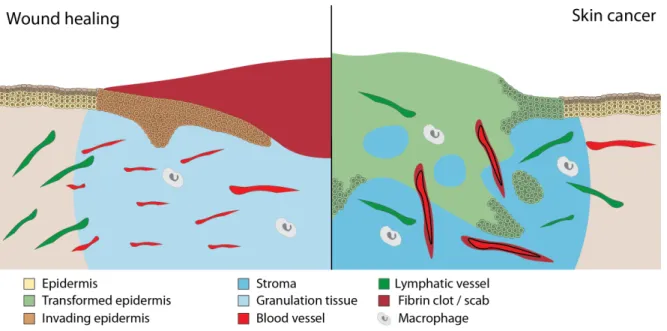
2.3. Cancer as an over-healing wound – similarities 32
2.3.1. The phases of wound healing 33
2.3.2. Cancer as an over-healing wound 37 2.3.3. Formation of new vessels in healing wounds and cancers 39
3. Aims of the thesis 41
4. Keratinocyte-expressed podoplanin is dispensable for tumor initiation
and growth in a model of chemically induced skin carcinogenesis 43
4.1. Abstract 44
4.2. Introduction 45
4.3. Materials and methods 47
4.4. Results 51
4.5. Discussion 59
5. Keratinocyte-expressed podoplanin is dispensable for re-epithelization but promotes lymphangiogenesis in cutaneous wound healing 63
5.1. Abstract 64
5.2. Introduction 65
5.3. Materials and methods 67
5.4. Results 73
5.5. Discussion 84
6. Conclusion and outlook 87
7. Acknowledgments 91
8. Abbreviations 92
9. References 95
1. Summary/Zusammenfassung 1.1. Summary
Podoplanin is a small transmembrane mucin-like glycoprotein. In adult tissues, it is expressed by a number of different cell types, including glomerular podocytes (giving its name), type I alveolar cells, mesothelial cells, choroid plexus cells, glia cells, different types of fibroblasts and lymphatic endothelial cells. Podoplanin is crucial for normal embryonic development and function of the lungs, the heart and the lymphatic vascular system, as demonstrated by studies in podoplanin knockout mice.
Podoplanin expression is upregulated in both epithelial and mesenchymal cell compartments during inflammation, wound healing and cancer, and a growing body of evidence indicates that it plays an important role in these pathologies.
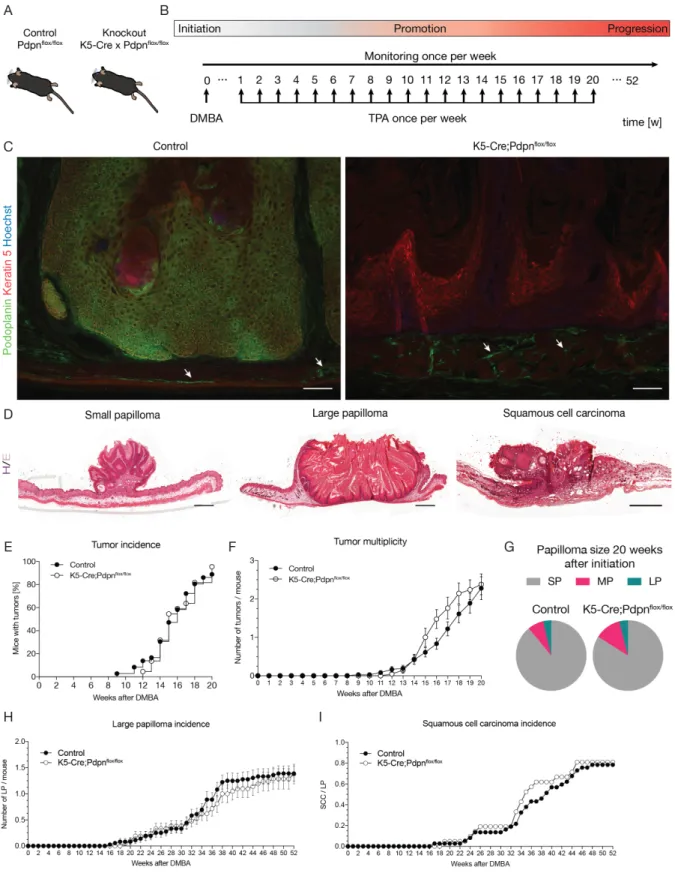
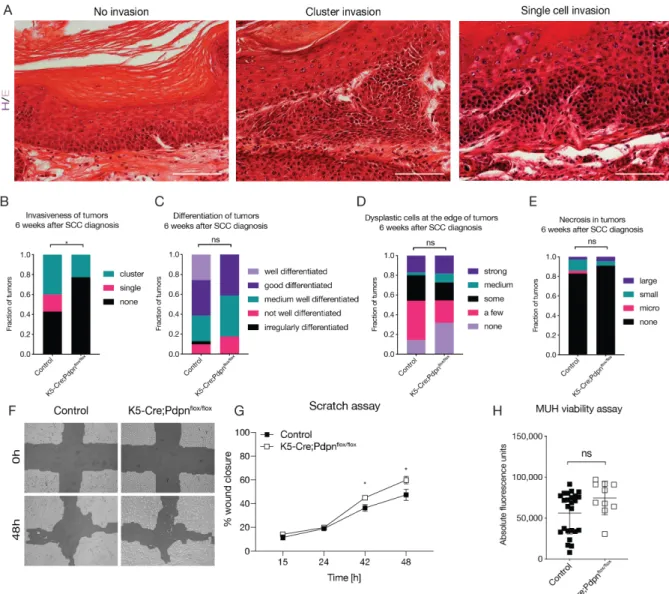
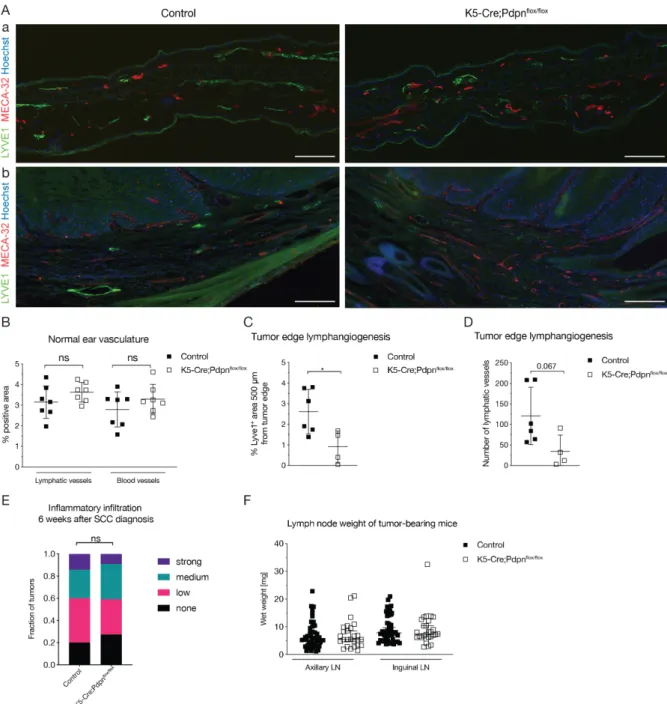
In the first project, we investigated the role of keratinocyte-expressed podoplanin in early skin carcinogenesis, as podoplanin expression in a number of different human carcinomas has been reported as a marker for poor prognosis and as a tumor promoter. To this end, we generated keratinocyte-specific podoplanin knockout mice and subjected them to an established orthotopic multistep skin carcinogenesis model. We found that keratinocyte-expressed podoplanin is dispensable for tumor initiation, multiplicity and malignant transformation. However, single cell tumor invasion was exclusively observed in control Pdpnflox/flox mice, while collective cell invasion was detected in both control and K5-Cre;Pdpnflox/flox mice. Quantification of the lymphatic vasculature revealed reduced peritumoral lymphangiogenesis in the K5Cre;Pdpnflox/flox mice, while there were no major changes of the immune cell infiltration.
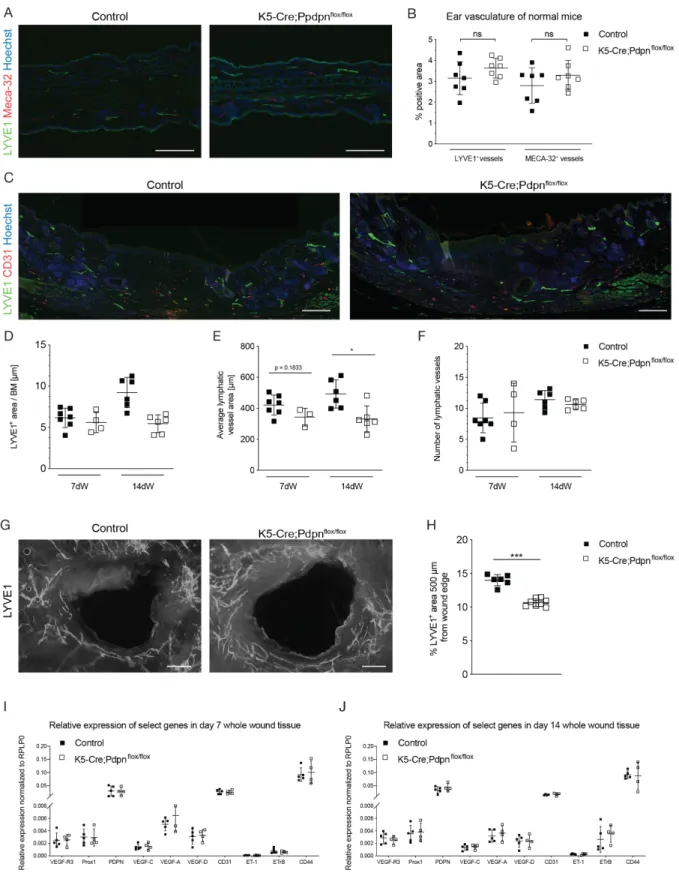
In the second project, we investigated the role of epidermal keratinocyte-expressed podoplanin in wound healing, as podoplanin expression is transiently upregulated during wound healing by keratinocytes and dermal cells. We found that keratinocyte- expressed podoplanin is dispensable for regular wound closure, re-epithelialization and maturation. However, abolition of keratinocyte-expressed podoplanin reduced
wound edge lymphangiogenesis, whereas there was an increased macrophage accumulation in the wounds of K5-Cre;Pdpnflox/flox mice.
In conclusion, keratinocyte-expressed podoplanin is dispensable for early carcinogenesis and wound closure. However, it appears to play an important role in cancer-associated and wound-associated lymphangiogenesis. Overall, these findings suggest that the role of podoplanin is versatile and cell-type- and disease- specific.
1.2. Zusammenfassung
Podoplanin ist ein kleines Transmembran-Mucin-ähnliches Glykoprotein. In adulten Geweben wird es von einer Reihe verschiedener Zelltypen exprimiert, wie z. B.
glomerulären Podozyten (woher der Name Podoplanin stammt), Alveolarzellen vom Typ I, Mesothelzellen, Plexuszellen des Plexus choroideus, Gliazellen, verschiedenen Arten von Fibroblasten und von lymphatischen Endothelzellen. Podoplanin ist essentiell für die normale Embryonalentwicklung und Funktion der Lunge, des Herzens und des lymphatischen Gefässsystems, wie Studien an Podoplanin- Knockout-Mäusen gezeigt haben. Die Podoplaninexpression ist sowohl in epithelialen als auch in mesenchymalen Zellen in Entzündungen, heilenden Wunden und Tumoren hochreguliert, und eine wachsende Zahl von Hinweisen zeigt, dass Podoplanin bei diesen Pathologien eine wichtige Rolle spielt.
Im ersten Projekt untersuchten wir die Rolle von Keratinozyten-exprimiertem Podoplanin in der frühen Karzinogenese der Haut, da die Podoplaninexpression in einer Reihe verschiedener menschlicher Karzinome als Marker für eine schlechte Prognose und als Onkogen beschrieben wurde. Zu diesem Zweck erzeugten wir Keratinozyten-spezifische Podoplanin-Knockout-Mäuse und unterzogen sie einem etablierten orthotopen mehrstufigen Hautkarzinogenese-Modell. Wir fanden heraus, dass Keratinozyten-exprimiertes Podoplanin für die Tumorinitiierung, Multiplizität und maligne Transformation entbehrlich ist. Jedoch wurde eine Einzelzellinvasion von Tumorzellen ausschliesslich bei Kontroll-Pdpnflox/flox-Mäusen beobachtet, während eine kollektive Zellinvasion sowohl bei Kontroll- als auch bei K5-Cre;Pdpnflox/flox- Mäusen nachgewiesen wurde. Die Quantifizierung der Lymphgefässe ergab eine verringerte peritumorale Lymphangiogenese bei den K5Cre;Pdpnflox/flox-Mäusen, während es keine wesentlichen Änderungen der Infiltration von Immunzellen gab.
Im zweiten Projekt untersuchten wir die Rolle von Keratinozyten-exprimiertem Podoplanin bei der Wundheilung, da die Podoplaninexpression während der Wundheilung sowohl durch Keratinozyten als auch durch mesenchymale Zellen vorübergehend hochreguliert wird. Wir fanden heraus, dass Keratinozyten-
exprimiertes Podoplanin für den Wundverschluss, die Reepithelialisierung und die Wundreifung entbehrlich ist. Jedoch war die Lymphangiogenese am Wundrand in K5- Cre;Pdpnflox/flox-Mäusen reduziert, während wir eine erhöhte Akkumulation von Makrophagen in den Wunden von K5-Cre;Pdpnflox/flox-Mäusen fanden.
Zusammenfassend lässt sich sagen, dass Keratinozyten-exprimiertes Podoplanin für die frühe Karzinogenese der Haut und den Wundverschluss entbehrlich ist. Es scheint jedoch eine wichtige Rolle für die Lymphangiogenese in Tumoren und Hautwunden zu spielen. Diese Befunde legen nahe, dass die Rolle von Podoplanin vielseitig und zelltyp- und krankheitsspezifisch ist.
2. Introduction
2.1. The lymphatic vasculature
Two vascular systems
The circulatory system comprises the circular blood vascular system and the linear lymphatic vascular system. The blood vasculature is responsible for transport of oxygen and nutrient distribution through the entire body, while the lymphatic vasculature’s main tasks are fluid homeostasis, initiation of immune responses and uptake of dietary lipids from the small intestine. The blood vasculature is a closed circulatory system with the heart as a central pump providing the driving force, while the lymphatic system is a blind-ended, unidirectional system.
2.1.1. Anatomy of the lymphatic vasculature
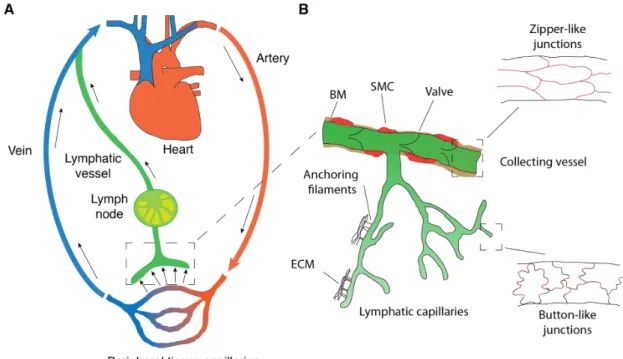
The lymphatic vasculature consists of hierarchically structured specialized components. The lymphatic vasculature begins with blind-ended capillaries, which are structurally optimized to drain fluid and cells (Figure 1). Lymphatic capillaries are formed by oak leaf-shaped lymphatic endothelial cells (LECs), which are connected by button-like junctions composed of vascular endothelial cadherin (VE-cadherin) and additional tight-junction proteins such as claudin-5 and occludin (Baluk et al., 2007; Baluk et al., 2005). Lymphatic capillaries are surrounded by a discontinuous basement membrane and mostly not covered by smooth muscle cells and pericytes (Alitalo, 2011). This construction makes initial lymphatic vessels highly permeable for signaling molecules, leukocytes and interstitial fluid. Since lymphatic capillaries lack a rigid structure, they are attached to the extracellular matrix (ECM) by anchoring filaments to prevent vessel collapse (Leak and Burke, 1968). The lymph is drained from the lymphatic capillaries to the pre-collectors, which have some smooth muscle cell coverage, into the collecting lymphatic vessels. In contrast to the capillaries, the collecting vessels are anatomically optimized for efficient fluid transport. Endothelial cells in the collectors are connected by continuous zipper-like junctions, covered with smooth muscle cells, and have a continuous basement membrane, resulting in a tight
vessel structure (Dejana et al., 2009). Finally, collectors have valves, consisting of two leaflets similar to veins, to prevent backflow of lymph (Alitalo, 2011) (Figure 1B). The lymph in the collecting vessels is driven by a number of mechanisms, including contraction of the smooth muscle cells encapsulating the collecting vessels, nearby skeletal muscles, as well as the pulsation of arteries.
Figure 1: Circulatory system and architecture of the lymphatic vasculature. (A) Schematic of the blood and lymphatic vascular systems. The heart drives the blood through the arteries and capillaries to the peripheral tissue, delivering oxygen and nutrients. The venous system returns deoxygenated blood (blue) back to the heart. The extravasated fluid, consisting of macromolecules and cells, is returned to the blood circulation by the lymphatic system (green). (B) Lymphatic vessel anatomy. The lymphatic system begins with oak leaf-shaped lymphatic endothelial cells, connected by the button like junctions, supporting fluid entry.
Lymphatic capillaries lack valves and coverage by smooth muscle cells (SMCs). To prevent collapse, the capillaries are anchored to the ECM by anchoring filaments. Collecting lymphatics have zipper-like junctions and are covered by a continuous basement membrane (BM), SMCs and contain valves to ensure unidirectional flow. Adapted from (Cueni and Detmar, 2006; Schwager et al., 2018; Zgraggen, 2014).
From the collecting vessels, the lymph is drained through the lymph nodes (LNs), which are the main site of immune surveillance. Lymph nodes are comprised of lobules surrounded by fluid-filled sinuses enclosed by a capsule. The outermost part of the lobules is the superficial cortex where B cell follicles are located. The inner part of the lobule is the paracortex or deep cortex housing naïve T cells. At the base of the lobule are the medullary cords housing plasma cells, memory T cells and macrophages, as well as blood vessels and nerves. The central-most sinuses are the medullary sinuses, which connect to the efferent lymphatic vessels. The paracortex also houses high endothelial venules (HEVs), which are blood vessels with a distinct morphology enabling B and T lymphocyte entry into the lymph node (Willard-Mack, 2006). Finally, the subcapsular sinus, the space between the lymph node capsule and the cortex, is where soluble antigens and antigen-presenting cells enter the lymph nodes via the afferent vessels. The subcapsular sinus is lined with fibroblastic reticular cells (FRCs) and specialized macrophages, which sample the drained lymph and acquire soluble antigens for presentation (Asano et al., 2011; Junt et al., 2007).
Next, the lymph flows along the cortex and paracortex, and into the medullary sinuses. Along the route of drainage, the soluble antigens may be recognized by B cells and initiate an antibody-based humoral immune response. Additionally, a cellular immune response may be triggered if the soluble antigen encounters and activates a naïve T lymphocyte. The lymph leaves the lymph node via the efferent lymphatics and the thoracic duct, before finally joining the blood circulation via the subclavian vein (Foster, 1996; Jeltsch et al., 2003).
The study of the lymphatic system has been greatly facilitated by the identification of lymphatic endothelial cell-specific markers, such as prospero homeobox 1 (PROX1), podoplanin (Pdpn), vascular endothelial growth factor receptor-3 (VEGFR-3) and lymphatic vessel endothelial hyaluronan receptor-1 (LYVE1) (Banerji et al., 1999;
Breiteneder-Geleff et al., 1999; Kaipainen et al., 1995; Wigle and Oliver, 1999).
Identification of these markers allowed for discrimination of lymphatic vessels from blood vessels, and subsequent detailed characterization (Cueni and Detmar, 2006).
Furthermore, expression of these lymphatic-specific markers in adulthood differs slightly and allows for the differentiation of collectors and capillaries, with collectors
lacking LYVE1 expression and valves having high PROX1 and VEGFR-3 expression compared to the collecting vessels (Mäkinen et al., 2005; Norrmen et al., 2009).
2.1.2. Development of the lymphatic vasculature Embryonic development of lymphatic vessels
The development of the lymphatic system has been a topic of research for more than a century. At the beginning of the 20th century, two competing theories were proposed for the embryonic origin of endothelial cells: the “centrifugal” and the “centripetal”
theory. The “centrifugal” theory postulated that lymphatic vessels sprout from the embryonic veins (Sabin, 1902). The “centripetal” theory postulated that lymphatic vessels develop from mesenchymal cells independently of blood vessels (Huntington and McClure, 1910). Lineage tracing experiments in mice with labeled PROX1 expressing cells showed that lymphatic vasculature originates from the venous cells, thus confirming the “centrifugal” theory (Srinivasan et al., 2007). Yet the question of the origin of lymphatic vessels has not yet been completely resolved, as evidence for both theories has been reported, as well as a compromise proposing a dual origin (i.e. venous and non-venous) (Semo et al., 2016). However, the current consensus favors a predominantly venous origin of lymphatic vessels and, consequently, this is presented in this section.
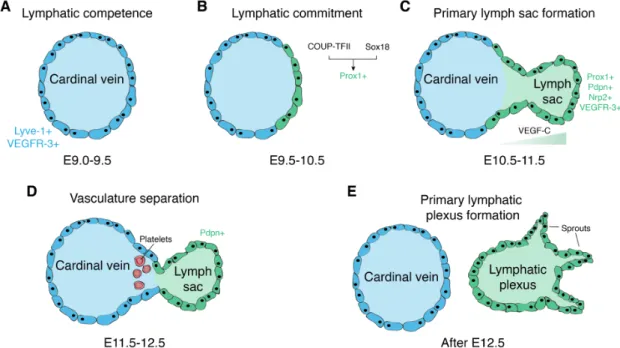
The development of the lymphatic vasculature begins after the blood vasculature has already been established (Figure 2A). In mice, the lymphatic development starts around embryonic day 9.5 (E9.5), after PROX1 is upregulated in a subset of endothelial cells in the cardinal vein (Figure 2B). PROX1 is a master regulator of lymphatic fate commitment, and has been shown to be sufficient to direct the endothelial cells towards the lymphatic fate both in vivo and in vitro (Hong et al., 2002a; Kim et al., 2010). Indeed, PROX1 is able to reprogram the transcriptional profile of blood vascular endothelial cells to more resemble lymphatic-specific expression and global PROX1 knockout mice completely lack any lymphatic vessels (Petrova et al., 2002; Wigle and Oliver, 1999). PROX1 itself is induced by the transcription factor sex determining region Y (SRY-) box 18 (Sox18), which is first
expressed at E8.5 (François et al., 2008). Sox18 knockout mice develop edema due to impaired lymphatic vessel development (François et al., 2008). The signaling cascade leading to the upregulation of Sox18 and, thereby, initiation of lymphatic differentiation, is still unknown. Another important component is the chicken ovalbumin upstream promoter-transcription factor II (COUP-TFII), which directly induces PROX1 expression. Later, PROX1-COUP-TFII interaction is necessary for the maintenance of LEC-specific gene expression (Srinivasan et al., 2010). Around E10.5, the PROX1 positive cells begin to bud off from the cardinal vein (Figure 2C). These lymphatic progenitor cells are connected by the adherens junctions containing VE- cadherin, and begin to express podoplanin and VEGFR-3 (Schacht et al., 2005).
VEGFR-3 is important, as the mesenchyme provides guidance cues via a dorsolateral gradient of its ligand, vascular endothelial growth factor-C (VEGF-C) (Karkkainen et al., 2004). Recently, the VEGF-C/VEGFR-3 axis has been shown to regulate both the expression of PROX1 in a positive feedback loop and the transcription factor MAFB, which is involved in lymphatic sprouting (Dieterich et al., 2015; Srinivasan et al., 2014).
The VEGF-C/VEGFR-3 axis is crucial for the physiological lymphatic development. In VEGF-C knockout mice, progenitor cells commit to the lymphatic fate, but fail to sprout from the cardinal vein (Karkkainen et al., 2004). VEGFR-3 knockout mice die between E10 and E12.5 before the lymphatic vasculature is established, due to cardiovascular failure, as VEGFR-3 is also expressed in blood vessels during embryonic development (Dumont et al., 1998). Under homeostatic conditions, the biological activity of VEGF-C is regulated by the collagen- and calcium-binding EGF domains 1 (CCBE-1). CCBE-1 was shown to be critical for the budding of PROX1 positive cells from the cardinal vein, but has little direct effects on LECs (Bos et al., 2011). Rather, it enhances the proteolytic processing of interlineated, inactive VEGF- C to its active form (Jeltsch et al., 2014). By E11.5, the migrating lymphatic progenitor cells establish the primary lymph sac. The next crucial step in the development of the lymphatic vasculature is the separation of the lymph sac from the cardinal vein (Figure 2D). This separation is mediated by the platelets accumulating between the cardinal vein and the lymph sac. The platelet expressed C-type lectin-like receptor-2 (CLEC- 2) interacts with LEC expressed podoplanin, activating the SYK and SLP-76 signaling
cascade, leading to platelet aggregation and sealing off the connection between the lymph sac and the cardinal vein (Bertozzi et al., 2010; Uhrin et al., 2010). In support of this, mice lacking SYL and SLP-76 show blood-filled lymphatics and impaired separation of the two vascular systems (Abtahian et al., 2003). A similar phenotype is observed in CLEC-2 knockout mice (Suzuki-Inoue et al., 2010). Subsequent sprouting from the lymph sacs results in the formation of the primary lymphatic plexus, from which the entire lymphatic vascular system is derived through maturation and differentiation into capillaries, pre-collectors and collectors (Figure 2E).
Figure 2: Development of the lymphatic vasculature. Overview of the milestones in the embryonic development of lymphatic vessels. (A) The cardinal vein initially consists of endothelial progenitors that have not yet committed to the lymphatic fate by E9.5. (B) Starting on E9.5, the transcription factors COUP-TFII and Sox18 upregulate PROX1, committing the endothelial progenitors to the lymphatic fate. (C) Lymphatic progenitor cells upregulate a number of other lymphatic-specific genes and start to bud off from the cardinal vein, guided by the VEGF-C gradient. (D) LEC-expressed podoplanin (Pdpn) and platelet-expressed CLEC-2 interaction separates the cardinal vein and the lymph sac. (E) Lymph sac sprouting establishes the primary lymphatic plexus, the origin of the eventual lymphatic system.
Adapted from (Cueni and Detmar, 2008; Schwager et al., 2018; Tammela and Alitalo, 2010).
The formation of the valves is a crucial step in the maturation of collecting lymphatic vessels; it begins with the upregulation of the transcription factor forkhead box C2 (Foxc2) starting around E14.5 to E15.5. As the collector LECs mature, they downregulate the expression of PROX1, LYVE1 and VEGFR-3. In a subset of cells in the collectors, the levels of PROX1 and FoxC2 remain high. These cells reorient themselves to protrude into the lumen of the lymphatic vessels and elongate to form the valve leaflets. This process is driven by Foxc2 in cooperation with calcineurin and the transcription factor nuclear factor of activated T cells, cytoplasmic-1 (NFATc1), all of which are expressed in the developing LECs (Norrmen et al., 2009). The PROX1 and Foxc2-dependent activation of the calcineurin/NFATc1 signaling pathway was shown to be a mechanosensory response to shear stress, which is the main valve- initiation stimulus (Sabine et al., 2012). Furthermore, the expression zinc finger transcription factor GATA2 is induced by the oscillatory flow and was suggested to directly regulate PROX1 transcription, making it essential for initiation of valve development (Kazenwadel et al., 2015). Finally, semaphorin 3A has been suggested to repel smooth muscle cells from the valve region, an important step in valve development (Bouvrée et al., 2012; Jurisic et al., 2012). During leaflet formation, extracellular matrix components such as fibronectin and laminin, are deposited in the center of the forming valves, and the endothelial cells upregulate integrins (e.g.
integrin-α9), increasing the attachment to the substrate. This ultimately results in the formation of functional valves consisting of an endothelial cell bilayer sandwiching a core of extracellular matrix (Bazigou et al., 2009).
2.1.3. Lymph node development
Around E12.5, lymph node development is initiated as the connective tissue and mesenchymal cells start protruding into the lymph sacs, generating the first lymph node anlagen. A critical step in the development of the lymph nodes is the maturation and recruitment of lymphoid tissue inducer (LTi) cells, a process dependent on the tumor necrosis factor-related activation-induced cytokine (TRANCE) and the retinoic- acid-receptor-related orphan nuclear receptor γ (RORγt) (Eberl et al., 2004; Kim et al., 2000). These LTis express lymphotoxin α1β2 and engage with stromal cells (so called
mesenchymal organizer cells) expressing the cognate lymphotoxin β receptor (LTβR) (Honda et al., 2001). This leads to the formation of cell clusters of inducer and organizer cells, and induces nuclear factor κB (NF-κB) signaling, resulting in the expression of adhesion molecules like intercellular adhesion molecule-1 (ICAM-1) and vascular cell adhesion molecule-1 (VCAM-1), as well as VEGF-C and several chemokines, such as C-X-C motif chemokine ligand 12 (CXCL12), CXCL13, C-C motif chemokine ligand 19 (CCL19) and CCL21 (Dejardin et al., 2002; Vondenhoff et al., 2009). These adhesion molecules facilitate cell adhesion, cell cluster formation and spatial organization. In turn, blood vessels mature into high endothelial venules (HEVs), while the chemokines attract lymphocytes into the developing node and are involved in establishing B and T cell zones. Finally, lymphangiogenesis and the lining of nodal sinuses with lymphatic endothelial cells is induced by VEGF-C (Blum and Pabst, 2006). These processes culminate in the formation of the mature and fully functioning lymph node.
2.1.4. Main lymphatic growth factors and signaling pathways
The lymphatic vascular system is generally static in the adult organism under physiological conditions. Only the placenta, the ovaries and the endometrium are the exceptions. However, the lymphatic vasculature may undergo dynamic remodeling in certain pathologies, such as cancer, inflammation and wound healing. Although the formation of new lymphatic vessels (lymphangiogenesis) is mediated by a variety of factors, the most important signaling axes are the vascular endothelial growth factors (VEGFs) and their cognate receptors (VEGFRs). Presently, it is unclear whether lymphangiogenesis is an exclusive result of local LEC proliferation and sprouting, or whether it also involves the incorporation of circulating endothelial progenitor cells into the sites of active lymphangiogenesis. Putative lymphatic endothelial progenitor cells co-expressing both lymphatic endothelial and stem cell markers have been identified in human fetal liver and cord blood (Salven et al., 2003).
However, in sublethally irradiated mice grafted with GFP-expressing bone marrow- derived endothelial progenitor cells, these cells did not contribute to tumor- or VEGF- C-induced lymphangiogenesis (He et al., 2004). Conversely, bone marrow-derived
cells were reported to be incorporated into the corneal lymphatics of inflamed or fibroblast growth factor 2 (FGF-2) treated corneas of GFP chimeric mice, as well as during inflammation-associated lymphangiogenesis in human renal transplants (Kerjaschki et al., 2006; Maruyama et al., 2005; Religa et al., 2005). However, these cells were suggested to be macrophages transdifferentiated into LECs and their overall contribution seems to be minor (Kerjaschki et al., 2006; Maruyama et al., 2005). Macrophages and other inflammatory cells may rather play an indirect role in pathological lymphangiogenesis by secreting lymphangiogenic factors such as VEGF-C or VEGF-D (Cursiefen et al., 2004; Schoppmann et al., 2002).
VEGF-C and VEGF-D are the most important and best characterized lymphangiogenic growth factors. Overexpression of VEGF-C in the skin induces hyperplasia of cutaneous lymphatic vessels (Jeltsch et al., 1997; Veikkola et al., 2001).
VEGF-C also promotes survival, migration and growth of cultured human LECs (Makinen et al., 2001). The principal receptor for both VEGF-C and VEGF-D is VEGFR- 3, and after proteolytic cleavage they can also bind to VEGFR-2 (Achen et al., 1998;
Joukov et al., 1996; Joukov et al., 1997; Yamada et al., 1997). The activation of VEGFR-3 signaling is sufficient to induce lymphangiogenesis, while the role of VEGFR-2 is less clear (Veikkola et al., 2001). To this end, several studies have shown the lymphangiogenic role of VEGF-A, a ligand of VEGFR-2 but not VEGFR-3, in vivo and in vitro (Hirakawa et al., 2007; Hirakawa et al., 2003; Hong et al., 2004a; Nagy et al., 2002). An indirect mechanism whereby VEGF-A attracts VEGF-C and -D- producing inflammatory cells has been proposed (Baluk et al., 2005; Cursiefen et al., 2004). However, at least part of this effect can be attributed to VEGFR-2 since it can be abolished by antibody blocking of VEGFR-2 (Hong et al., 2004a; Kunstfeld, 2004).
Alternatively, VEGFR-2 was suggested to promote lymphatic vessel enlargement, but not sprouting (Wirzenius et al., 2007). In support of this, in a model of lymphangiogenesis in regenerating skin, both VEGFR-2 and VEGFR-3 were required for LEC migration and proliferation, while either was sufficient for the subsequent organization of LECs into functional capillaries (Goldman et al., 2007). Interestingly, VEGFR-3 signaling in mice is only required for lymphatic vessel maintenance during the first few weeks of life; thereafter, the lymphatic vessels can regenerate
independent of VEGFR-3 signaling (Karpanen et al., 2006; Pytowski et al., 2005).
These results suggest that other factors might become important for lymphangiogenesis and lymphatic vessel maintenance in adult organisms. Indeed, a number of factors have been reported to promote lymphangiogenesis, including angiopoietin-1 (Ang-1), hepatocyte growth factor (HGF), fibroblast growth factor 2 (FGF-2), insulin-like growth factor-1 (IGF-1), platelet derived growth factors (PDGF) and adrenomedullin (AM) (Bjorndahl et al., 2005; Cao et al., 2004; Chang et al., 2004b;
Fritz-Six et al., 2008; Kajiya et al., 2005; Kubo et al., 2002; Morisada et al., 2005)
2.1.5. Lymphatic vessels in inflammation
Inflammation is a defensive reaction of the organism that occurs as a response to tissue injury, infection, in the context of autoimmune diseases and during tumor growth. In inflammation, the lymphatic vessels have traditionally been regarded as a passive drainage system transporting leukocytes, inflammatory mediators and antigens. However, in recent years, it has become increasingly clear that lymphatic vessels have an active role in inflammatory processes and modulate immune responses.
During inflammation, the lymphatic vessels quickly alter their gene and protein expression in an inflammatory stimulus dependent fashion. Adhesion molecules are one important class of affected molecules. Inflammation causes upregulation of ICAM-1, VCAM-1, E-selectin, P-selectin and activated leukocyte cell adhesion molecule (ALCAM), involved in leukocyte trafficking (Iolyeva et al., 2013; Johnson et al., 2006; Vigl et al., 2011). Activated LECs express E- and P-selectins that bind glycosylated proteins via their carbohydrate-recognizing domains, mediating leukocyte adhesion by binding their ligands E-selectin ligand-1 (ESL-1) and/or P- selectin glycoprotein ligand-1 (PSGL-1) presented on the surface of leukocytes (Muller, 2013). LEC expressed ICAM-1 and VCAM-1 interact with the leukocyte expressed integrins, leukocyte function-associated antigen-1 (LFA-1) and very late antigen-4 (VLA-4), enabling crawling on the endothelial cells, ultimately resulting in transmigration of immune cells into and migration in the lymphatic vessels. These
processes play a key role in efficient migration of antigen-presenting cells such as dendritic cells (DCs) from the periphery to the lymph node. In the lymph node, DCs encounter and activate T cells (Teijeira et al., 2013). Antibody-mediated blocking of ICAM-1, VCAM-1 or the ligand LFA-1 in inflammation results in a striking reduction of DC migration to the lymph node (Johnson et al., 2006; Nitschké et al., 2012; Teijeira et al., 2013). Another set of molecules subject to inflammation-induced upregulation in LECs are the chemokines. CCL21 is the best studied representative, attracting T cells, DCs and neutrophils expressing the C-C chemokine receptor 7 (CCR7) (Beauvillain et al., 2011; Brown et al., 2010). Interestingly, CCL21 is stored in intracellular vesicles and rapidly released upon stimulation with inflammatory mediators such as tumor necrosis factor-α (TNF-α) (Johnson and Jackson, 2010). In addition to CCL21, numerous other chemokines are also upregulated during inflammation, such as CXCL12 which supports migration of cutaneous dendritic cells (i.e. Langerhans cells and dermal DCs) expressing the CCR4 receptor to draining lymph nodes (Kabashima et al., 2007). Some chemokines are pro-lymphangiogenic, such as C-X-C motif chemokine ligand 8 (CXCL8, also called interleukin (IL)-8) and CXCL12 (Choi et al., 2013), while others are anti-lymphangiogenic, such as CXCL10 and CXCL11 (Nielsen et al., 2013; Zhuo et al., 2012). Intriguingly, the lymphatic vessel markers PROX1, LYVE1 and VEGFR-3 are downregulated under certain inflammatory conditions such as ultraviolet B irradiation and oxazolone sensitization, but not others like complete Freund’s adjuvant or thioglycolate (Flister et al., 2010; Huggenberger et al., 2011; Vigl et al., 2011).
Additionally, the morphology of lymphatic vessels is also altered in inflammation, most notably the enlargement of vessels (Kunstfeld, 2004). Lymphatic vessels undergo extensive expansion in a number of inflammatory diseases such as psoriasis, chronic airway inflammation, inflammatory bowel disease and rheumatoid arthritis (Baluk et al., 2005; Jurisic et al., 2013; Kunstfeld, 2004; Wauke et al., 2002).
This expansion is mediated by VEGF-A and VEGF-C secreted by the keratinocytes, stromal cells and immune cells, most importantly the macrophages (Cursiefen et al., 2004; Kim et al., 2009). The critical importance of macrophages was demonstrated in a model of lipopolysaccharide (LPS)-induced skin inflammation, where depletion
of macrophages delayed inflammation resolution and reduced lymphangiogenesis (Kataru et al., 2009).
Morphological changes to the lymphatic vessels are not only limited to vascular dilation and expansion, but may also affect cellular junctions and lymphatic drainage function. In chronic airway inflammation, the lymphatic capillaries replace their characteristic button-like junctions with continuous zipper-like junctions, possibly affecting lymphatic barrier and drainage function (Yao et al., 2012). On the other hand, there are conflicting reports on whether inflammation enhances or compromises lymphatic drainage function. Organ-specific inflammatory effects may account for at least part of these discrepancies, as LPS-induced inflammation of the skin improves, while LPS-induced inflammation of peritonitis negatively impacts lymphatic drainage (Kataru et al., 2009; Kim et al., 2009). Additionally, the duration of inflammation may also play a role. In acute skin inflammation, lymphatic drainage is not significantly affected, while chronic inflammation significantly reduces lymphatic drainage (Huggenberger et al., 2011).
The role of the expanded lymphatic endothelium in inflammation has been controversial. On one hand, it was suggested that the expansion of the lymphatic endothelium contributes to inflammation by facilitating transport of leukocytes to lymph nodes and mounting of immune responses, while others have suggested that lymphatic vessels support inflammation resolution by draining inflammatory mediators and cells from the site of inflammation. In response to this, a number of studies have investigated the effects of VEGF-C-mediated lymphatic vasculature expansion on disease severity in models of rheumatoid arthritis, inflammatory bowel disease and several models of skin inflammation (Christiansen et al., 2016; D’Alessio et al., 2014; Huggenberger et al., 2010; Kajiya et al., 2009; Zhou et al., 2011). All of these studies are in agreement that lymphatic vasculature expansion alleviated inflammation, indicating that promoting lymphatic expansion supports inflammation resolution and may represent a valid therapeutic approach.
2.1.6. Lymphatic vessels in cancer
Similar to immune cells, cancer cells use the lymphatic system for trafficking through the body. Lymph node metastasis represents the first step in tumor dissemination and is an important prognostic marker for disease progression. Meanwhile, it is a well-established concept that tumors actively induce tumor-associated lymphangiogenesis by secreting factors such as VEGF-C, VEGF-D or VEGF-A, rather than just accidentally invading pre-existing lymphatic vessels (Hirakawa et al., 2007;
Hong et al., 2004a; Skobe et al., 2001; Stacker et al., 2001). Tumor-induced lymphangiogenesis promotes the spread of cancer cells to the draining (sentinel) lymph nodes and beyond. Tumor-derived factors, such as VEGF-A and VEGF-C, have been found to also induce expansion of the lymphatic network in the sentinel lymph nodes, even before the arrival of the metastatic cancer cells, presumably to create a favorable environment for the arriving cancer cells (Hirakawa et al., 2007;
Hirakawa et al., 2005). Metastatic tumor cells in the sentinel lymph nodes continue to induce lymphangiogenesis within the lymph node, increasing the drainage and inducing lymphangiogenesis in the distant lymph nodes. VEGF-C-induced lymph node lymphangiogenesis was shown to promote both tumor metastasis to organs and distant lymph nodes in squamous cell carcinomas (Hirakawa et al., 2007).
Recently, it was found that lymphangiogenesis also occurs in distant organ metastases and that this is associated with reduced patient survival (Ma et al., 2018).
Overall, tumor-associated lymphangiogenesis, sentinel lymph node lymphangiogenesis and distant lymph node metastasis are indicators for a poor prognosis (Dadras et al., 2005; Dadras et al., 2003; Rinderknecht and Detmar, 2008).
Taken together, targeting lymphangiogenesis in the primary tumor or the draining lymph nodes might be a strategy to prevent tumor metastasis. To this end, the most promising strategy appears to be stalling the action of VEGF-C and VEGF-D by blocking the crucial lymphangiogenic signaling of VEGFR-3.
2.1.7. Lymphatic vessels in wound healing
In dermal wound healing, the first step is the formation of a fibrin clot that is infiltrated by immune cells (i.e., neutrophils, monocytes, and macrophages) to be populated by anti-inflammatory macrophages and myofibroblasts. The appearance of myofibroblasts is followed by transient blood vessels that supply the wound with oxygen and nutrients for the support and surveillance of wound healing. The mechanisms of rapid restoration of blood circulation in wound healing are well understand and include tension-dependent looping angiogenesis with sprouting and intussusception (Kilarski and Gerwins, 2009; Kilarski et al., 2009). Even though it is well-established that lymphangiogenesis follows angiogenesis in wound healing, the mechanisms of lymphangiogenesis and the precise role of the lymphatics in the context of wound healing remain largely unknown (Baluk et al., 2013; Goldman et al., 2007; Norrmen et al., 2011; Paavonen et al., 2000; Rutkowski et al., 2006).
Interstitial fluid flow, such as that generated in inflamed or healing tissues, was shown to stimulate myofibroblast differentiation and matrix alignment (Ng and Swartz, 2003).
In turn, increased interstitial fluid flow was suggested to increase directional wound contractility and vascularization (Kilarski et al., 2012; Tomasek et al., 2002). Wound lymphangiogenesis was also speculated to alter immune cell trafficking from the wound, since LEC expressed molecules like E-selectin, ICAM-1 and LYVE1 regulate leukocyte emigration (Güç et al., 2017; Lawrance et al., 2016; Rigby et al., 2015).
These changes in leukocyte trafficking could alter the cytokine and growth factor signaling in the wound, thereby directly stimulating matrix remodeling (Güç et al., 2017). Reduced wound lymphangiogenesis was reported to directly contribute to impaired diabetic skin wound healing (Maruyama et al., 2007). Despite this, few studies have looked at the promotion of lymphangiogenesis as a therapy for chronic diabetic wounds (Saaristo et al., 2006; Szuba et al., 2002; Tammela et al., 2007).
Interestingly, adenoviral VEGF-C overexpression in diabetic skin wounds promoted wound healing predominantly via the activation of blood vessel angiogenesis and attraction of macrophages (Saaristo et al., 2006).
2.2. Podoplanin
Podoplanin (Pdpn) is a small type I transmembrane mucin-like glycoprotein known under many different names, including PA2.26, gp38, T1α, D2-40, and aggrus. It first appeared around 500 million years ago in jawed vertebrates, and is not present in jawless fishes and non-vertebrate chordates (Renart et al., 2018). In adult tissues, it is expressed in a number of different tissue and cell types, such as glomerular podocytes (hence its name), type I alveolar cells, mesothelial cells, choroid plexus cells, glia cells, sebaceous glands, different types of fibroblasts, and LECs. Global podoplanin knockout mice exhibit several developmental defects, suggesting its important role in embryonic development (Astarita et al., 2012; Renart et al., 2015;
Schacht et al., 2005). During inflammation and cancer, podoplanin is upregulated in both epithelial and mesenchymal compartments, and a growing body of evidence suggests that it plays an important role in these pathologies.
2.2.1. Podoplanin structure
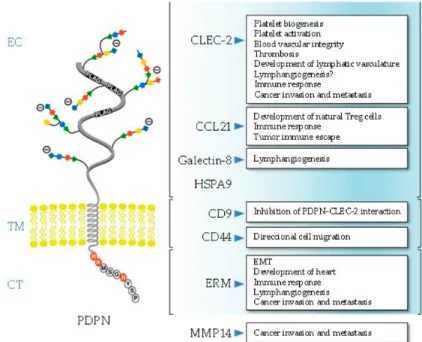
Podoplanin exhibits the typical structure of a type I transmembrane mucin-like glycoprotein, with a heavily O-glycosylated ectodomain, a hydrophobic membrane spanning domain, and a short cytoplasmic tail of only nine amino acids (Figure 3).
Ectodomain
The podoplanin ectodomain has a typical mucin O-glycan structure with galactose β3- linked to N-acetyl-galactosamine (GalNAc), called core 1 O-glycan, modified by addition of sialic acid (Martin-Villar et al., 2005; Scholl et al., 1999). Podoplanin- induced platelet aggregation and activation, one of the main biological functions of podoplanin discovered so far, is mediated by conserved platelet aggregation- stimulating (PLAG) motifs in the ectodomain (Navarro-Nunez et al., 2013; Suzuki- Inoue et al., 2017; Takemoto et al., 2017). Podoplanin induces platelet aggregation and activation through interaction with C-type lectin-like receptor 2 (CLEC-2) (Shin and Morita, 1998). Three tandem PLAG motifs mediate the platelet aggregation and
activation; the O-glycan at T-52 in the human PLAG3 domain and the O-glycan at T- 34 in mouse PLAG1 appear to be critical (Kaneko et al., 2006; Kato et al., 2003).
Additionally, podoplanin-induced platelet aggregation is important for the correct separation of the lymphatic and blood vasculatures during development and in cancer metastasis (Navarro-Nunez et al., 2013; Takemoto et al., 2017). Another glycosylation-dependent interaction partner of podoplanin is galectin-8, a tandem- repeat member of the galectin family (Cueni and Detmar, 2009). Galectin-8 has two carbohydrate recognizing domains that bind sialyl- and β-galactoside-containing glycans (Troncoso et al., 2014). Galectin-8 is also highly expressed in LECs and promotes pathological lymphangiogenesis via its interaction with podoplanin (Chen et al., 2016; Troncoso et al., 2014).
Figure 3: Schematic representation of the structure of human podoplanin. Highlighted are the main ligands and biological processes in which the interaction with podoplanin is involved. The main structural domains of podoplanin involved in ligand binding are indicated.
EC, ectodomain; TM, transmembrane region; CT, cytosolic domain. Adapted from (Quintanilla et al., 2019)
The ectodomain of podoplanin can also be shed from LECs into the perivascular stroma, and form complexes with the lymphatic-specific chemokine CCL21 and CCR7-positive immune cells, thereby promoting lymphangiogenesis (Kerjaschki et
al., 2004). This was suggested to be mediated by the release of podoplanin- containing lymphatic exosomes (Brown et al., 2018). The CCL21-podoplanin interaction has also been reported to aid tumor immune escape (Tejchman et al., 2017).
Transmembrane domain
The transmembrane region of podoplanin is highly conserved, particularly the N- terminal GXXXG region involved in helix-helix oligomerization (Fernandez-Munoz et al., 2011; Renart et al., 2018). While the functional relevance is unknown, podoplanin is a substrate of presenilin-1 (PS1)/γ-secretase, which cleaves the intracellular domain of podoplanin into the cytosol (Yurrita et al., 2014). Podoplanin’s transmembrane domain has also been reported to interact with CD9 tetraspanin and CD44 hyaluronan receptor (Martin-Villar et al., 2010; Nakazawa et al., 2008). Recently, it was reported that podoplanin-CD9 interaction inhibits the podoplanin-mediated platelet activation and podoplanin-induced metastasis (Nakazawa et al., 2008).
Conversely, the podoplanin-CD44 interaction promotes directional cell migration of squamous cell carcinoma (SCC) cells (Martin-Villar et al., 2010).
Cytoplasmic tail
The cytoplasmic tail of podoplanin is comprised of only nine amino acids. The first two residues are responsible for podoplanin binding to ezrin and moesin, which anchor it to the actin cytoskeleton (Martin-Villar et al., 2006). Ezrin and moesin belong to the ERM (ezrin, radixin, moesin) protein family. ERM proteins anchor the actin cytoskeleton to the cell membrane and are involved in cell polarity, adhesion and migration (Clucas and Valderrama, 2014). The podoplanin-ERM interaction was reported to modulate podoplanin-mediated cell migration via the Rho GTPases and epithelial–mesenchymal transition (EMT) during embryonic development and carcinogenesis (Krishnan et al., 2018; Renart et al., 2015). Furthermore, this interaction was reported to modulate lymphangiogenesis, immune response and podoplanin recruitment to the invadopodia (Astarita et al., 2012; Martin-Villar et al., 2015). The two serine residues of the cytosolic domain have been suggested as
putative phosphorylation sites. Phosphorylation of these two serines was reported to be under the control of protein kinase A (PKA) and cyclin-dependent kinase 5 (CDK5), resulting in inhibition of podoplanin-induced cell motility (Krishnan et al., 2013;
Krishnan et al., 2015).
2.2.2. Podoplanin in embryonic development and differentiation
Global podoplanin knockout mice die during embryonic development due to cardiovascular malformations or shortly after birth due to respiratory failure, owing to reduced expansion of respiratory alveoli. Cardiovascular malformations stem from enhanced E-cadherin expression and downregulation of RhoA GTPase, resulting in abnormal EMT of the coelomic epithelium (Mahtab et al., 2009; Mahtab et al., 2008).
Respiratory failure is due to deficient differentiation of type I alveolar cells, resulting in the inability to inflate the lungs at birth; the molecular mechanism behind this phenotype is unknown (Ramirez et al., 2003).
Blood-lymphatic vessel separation
The most striking phenotype of global podoplanin knockout mice are the blood-filled lymphatic vessels, edema and defective blood-lymphatic vessel separation (Schacht et al., 2003; Uhrin et al., 2010). The separation of the blood and lymphatic vascular systems occurs around embryonic day 11.5 (E11.5) in mice, mainly due to podoplanin-CLEC2 induced platelet activation. Podoplanin-CLEC2 binding induces downstream signaling pathways involving Src and SYK family tyrosine kinases, the adaptor protein SLP-76, and activation of PLCγ2 (Pollitt et al., 2014; Severin et al., 2011; Suzuki-Inoue et al., 2006; Suzuki-Inoue et al., 2007). Interestingly, the podoplanin-CLEC2 interaction is even able to induce platelet activation under arterial shear stress (Lombard et al., 2018). Podoplanin-CLEC2 mediated activation of the Syk/SLP-76 signaling pathway releases the platelet granule content, induces platelet aggregation and seals the separation zone of the lymphatic sacs and the cardinal vein (Bertozzi et al., 2010; Uhrin et al., 2010). Interestingly, a similar phenotype with blood-filled lymphatic vessels, edema and defective blood-lymphatic vessel
separation is also observed in mice lacking CLEC-2, PROX1 and endothelial glycosyltransferase T-synthase, which controls the mucin-type O-glycosylation (Bertozzi et al., 2010; Fu et al., 2008; Wigle et al., 2002). Podoplanin-CLEC2 activated platelets inhibit LEC proliferation and migration via the bone morphogenetic protein 9 (BMP-9), a member of a TGF-β growth factor subfamily (Osada et al., 2012). In adult tissues, podoplanin prevents blood backflow at the lympho-venous junction via the podoplanin-CLEC2 induced platelet aggregation (Suzuki-Inoue et al., 2017).
Cerebrovascular patterning and integrity
In addition to the blood-filled lymphatic vessels, edema and defective blood- lymphatic vessel separation, global podoplanin knockout mice also display a neurovascular phenotype. During development, podoplanin is expressed by the developing neural tube, and both podoplanin and CLEC-2 knockout mice show defects of neurovascular integrity in the embryonic brain, resulting in hemorrhaging after midgestation (Lowe et al., 2015). Neuroepithelial cell expressed podoplanin was shown to interact with CLEC-2 on the platelets that leak out of the surrounding brain blood vessels, inducing platelet activation and aggregation, thereby preventing hemorrhaging and promoting vessel maturation (Lowe et al., 2015).
Development of natural regulatory T-cells
Global podoplanin knockout mice also exhibit defects in the maturation of immune cells. Global podoplanin knockout mice have a delayed development of natural regulatory T (T reg) cells, resulting in hyperimmunoglobulinemia shortly after birth.
Natural T regs develop in the thymus as a subset of CD4+ cells and are important in the maintenance of self-tolerance and immune homeostasis (Sanchez and Yang, 2011). T reg development and maturation requires concentration of the CCL21 chemokine in the medullar area of the thymus. Podoplanin and CCL21 co-expression on thymic fibroblastic reticular cells (FRCs) appears to be critical for the correct localization of CCL21 within the thymus and the development and maturation of T regs (Fuertbauer et al., 2013).
In addition to the functions discussed here, podoplanin has also been reported to have an important role in mammary stem cell function, megakaryocyte growth and platelet production, and flattening of the podocyte foot (Bresson et al., 2018; Kojima et al., 2004; Shankland, 2006; Tamura et al., 2016).
2.2.3. Transcriptional control of podoplanin expression
The transcriptional control of podoplanin was first investigated in the context of the development of the lymphatic vasculature. Podoplanin is specifically expressed by the budding lymphatic sac, but not by the nearby endothelial cells of the cardinal vein. This observation lead to the discovery of PROX1 as a regulator of podoplanin expression (Hong et al., 2002b). Moreover, forced expression of PROX1 in differentiated BECs can induce an LEC-like phenotype, including upregulation of podoplanin (Hong et al., 2002b). However, podoplanin is expressed in a number of tissues where PROX1 is not expressed. This suggests an alternative regulation of podoplanin expression in tissues other than lymphatic endothelial cells. These alternative transcription controllers have been suggested as the reason why the physiological functions of podoplanin are so varied (Astarita et al., 2012)
In gliomas, osteosarcomas and certain skin cancers, podoplanin was reported to be regulated by the AP-1 transcription factor (Durchdewald et al., 2008; Kunita et al., 2011; Peterziel et al., 2012). AP-1 is a heterodimer consisting of Fos and Jun proteins, which are critical for the progression of many carcinomas including those developing in models of skin carcinogenesis (Eferl and Wagner, 2003). Keratinocyte-specific deletion of Fos downregulates podoplanin in a mouse model of chemical skin carcinogenesis (Durchdewald et al., 2008). Podoplanin expression was reported to be repressed by PTEN, a negative regulator of the PI3K-AKT-AP-1 pathway (Peterziel et al., 2012). Furthermore, under homeostatic conditions in tissues where podoplanin is not normally expressed, its promoter is heavily methylated, potentially contributing to the repression of expression (Peterziel et al., 2012).
In contrast to malignant conditions where podoplanin expression appears to be mainly under the control of the AP-1 transcription factor, alternative transcription regulators have been reported in the inflammatory setting. In rheumatoid arthritis (RA), the fibroblast-like synoviocytes are the main mediators of inflammation and tissue destruction (Huber et al., 2006). In healthy tissue, podoplanin is not expressed in the synovium, however, podoplanin was reported to be highly upregulated in the synovium of RA patients (Ekwall et al., 2011). Podoplanin was also reported to be upregulated in cultured synoviocytes upon treatment with IL-1β, TNF-α or TGF-β1 (Ekwall et al., 2011). Likewise, treatment of cultured keratinocytes with TGF-β, IL-6, IL-22 or IFN-γ was also reported to upregulate podoplanin (Honma et al., 2012b).
TGF-β-mediated podoplanin upregulation requires Smad2/3 and 4 signaling, and IFN-γ-mediated upregulation requires STAT1 and STAT3, whereas STAT1 is sufficient for IL-6- and IL-22-mediated upregulation of podoplanin (Honma et al., 2012a).
Overall, podoplanin expression appears to be regulated by a number of different stimuli, including differentiation factors during development and pro-tumorigenic and pro-inflammatory factors in pathologies. It is possible that distinct regulators of podoplanin expression can induce distinct downstream signaling pathways, leading to different cellular outcomes.
2.2.4. Podoplanin in pathology Podoplanin in the immune system
While podoplanin is a well-established marker of LECs and FRCs, it also plays a key role in the intravasation of DCs into afferent lymphatic vessels and DC migration to and within lymph nodes. Antigen-presenting DCs play a key role in the initiation of the immune response against foreign pathogens and in maintaining tolerance against self-antigens. Several mechanisms have been proposed on how podoplanin regulates DC migration. LEC expressed podoplanin immobilizes the chemokine CCL21 on the lymphatic endothelium. Then, CCL21 acts as a chemoattractant for migratory DCs, which concurrently upregulate the chemokine receptor CCR7,
promoting DC adhesion to the LEC endothelium and transmigration into the lumen of the vessel (Marsee et al., 2009).
Podoplanin in wound healing
In normal skin, podoplanin is expressed in lymphatic vessels, the basal cell layer of sebaceous glands and the outer root sheath of anagen hair follicles, but not in the interfollicular epidermis (Honma et al., 2012a; Martin-Villar et al., 2005). Interestingly, podoplanin expression in hair follicles correlates with the expression of the stem-cell marker CD34 and regulates hair follicle cycling (Yoon et al., 2019). Podoplanin expression is upregulated in the basal keratinocytes and dermal fibroblasts under hyperproliferative conditions, such as wound healing, psoriasis and inflammation induced by the phorbol ester 12-O-tetradecanoylphorbol 13-acetate (TPA) (Asai et al., 2016; Durchdewald et al., 2008; Honma et al., 2013; Honma et al., 2012a).
Podoplanin in keratinocytes is upregulated by pro-inflammatory cytokines such as TGF-β1, IFN-γ, interleukin 6 (IL-6) and IL-22 (Honma et al., 2012a). TGF-β1 was shown to induce podoplanin expression via the Smad pathway, while IFN-γ, IL-6, and IL-22 induce podoplanin expression via activation of the transcription factors STAT- 1 and STAT-3 (Honma et al., 2012a). Additionally, it was recently shown that overexpression of inhibitory protein of κB family kinase α (IKKα) can induce podoplanin expression (Alameda et al., 2016; Cho et al., 2012).
Podoplanin is transiently upregulated during wound healing and its expression correlates with re-epithelialization. Additionally, podoplanin positive keratinocytes at the wound edge downregulates E-cadherin, suggesting that podoplanin promotes keratinocyte migration rather than proliferation (Honma et al., 2013). In support of this, podoplanin knockdown experiments in mouse keratinocytes upregulated E-cadherin and inhibited migration (Asai et al., 2016). Conversely, overexpression of podoplanin in mouse keratinocytes induced cell migration and EMT without affecting proliferation (Scholl et al., 2000; Scholl et al., 1999). Interestingly, treating primary human keratinocytes with platelets or soluble CLEC-2 reduced cell migration, downregulated RhoA GTPase activity and upregulated E-cadherin (Asai et al., 2016), suggesting that
podoplanin-CLEC2 signaling inhibits keratinocyte migration in a similar fashion as it attenuates contractility in FRCs (Astarita et al., 2015). Therefore, platelet-keratinocyte interactions could be reducing keratinocyte motility during wound healing via the podoplanin-CLEC2 axis. Taken together, these results suggest a possible role for podoplanin during the initial phases of wound healing.
Podoplanin in cancer
Podoplanin expression is upregulated in a number of different cancers, including germ-cell tumors, gliomas, glioblastomas, osteosarcomas, malignant osteosarcomas and squamous cell carcinomas (SCCs) (Astarita et al., 2012; Renart et al., 2015;
Schacht et al., 2005; Ugorski et al., 2016) . Additionally, podoplanin is expressed by the stromal cells, particularly by cancer-associated fibroblasts (CAFs). Cancer cell expression of podoplanin has generally been reported as a poor prognostic marker, especially in glioblastomas and SCCs of skin, esophagus and head and neck (Astarita et al., 2012; Cueni et al., 2010; Renart et al., 2015) . Interestingly, podoplanin expression in SCCs of the uterine cervix and the lung was reported as a good prognostic factor (Dumoff et al., 2005; Ito et al., 2009; Suzuki et al., 2011). Podoplanin expression is often limited to the outer edge of tumors, suggesting that the stromal cells might be able to induce podoplanin expression in the transformed epidermis through secretion of cytokines and growth factors (Martin-Villar et al., 2005;
Rahadiani et al., 2010; Yuan et al., 2006). Supporting evidence for this assumption comes from a recent publication, showing that CD45+ cell-secreted pro-inflammatory cytokines can induce podoplanin expression at the invasive front of cervical SCC (Kunita et al., 2018). In addition to expression by the cancer cells themselves, a plasma soluble podoplanin has been reported as a poor prognostic marker in patients with adenocarcinoma, head and neck SCCs, and bladder, lung, gastric and rectal cancers (Sankiewicz et al., 2016; Zhao et al., 2018). Intriguingly, levels of soluble podoplanin were reduced after chemotherapy or surgery followed by chemotherapy, suggesting it as a potential cancer biomarker (Zhao et al., 2018). The origin of this soluble fraction is unknown. Several studies have reported that podoplanin’s ectodomain can be cleaved by different types of proteases, including calpains,
presenilin-1/γ-secretase and metalloproteases, and that its stability is regulated by O-glycosylation and sialylation (Pan et al., 2014; Yurrita et al., 2014). Alternatively, full length podoplanin has been found in microvesicles and exosomes in malignant pleural effusions and cancer cell line supernatants (Carrasco-Ramirez et al., 2016;
Roca et al., 2016).
Podoplanin expression in the stromal compartment
Cancer-associated fibroblasts (CAFs) represent a special type of activated fibroblasts incorporated into the tumor microenvironment. CAFs promote malignant progression of cancers by releasing growth factors, cytokines and matrix remodeling proteins, promoting angiogenesis, lymphangiogenesis, and tumor cell migration and invasion (Kalluri and Zeisberg, 2006). CAFs upregulate podoplanin in a number of cancers, including some in which podoplanin is not expressed by the cancer cells themselves such as adenocarcinomas of the breast, lung and pancreas (Pula et al., 2013).
Similarly, as with cancer cell expressed podoplanin, CAF expressed podoplanin in most cancers is associated with lymph node metastasis and shorter overall survival (Kitano et al., 2010; Pula et al., 2013). Interestingly, in colorectal and small-cell lung carcinomas, CAF expressed podoplanin has been reported as a favorable prognostic marker (Takahashi et al., 2013; Yamanashi et al., 2009). A number of studies have tried to understand the molecular mechanism of how podoplanin positive CAFs promote malignancies. In vitro, podoplanin expressing CAFs were reported to promote invasiveness of pancreatic carcinoma cell lines (Shindo et al., 2013).
Similarly, podoplanin expressing CAFs were shown to promote the tumor formation ability of human lung adenocarcinomas via the RhoA-ROCK signaling cascade (Ito et al., 2012; Neri et al., 2015). Podoplanin expressing CAFs were also suggested to promote cancer invasion and motility independently of matrix metalloproteinase (MMP) activity, and to mediate EGFR inhibitor resistance in patients with lung adenocarcinomas (Neri et al., 2015; Yoshida et al., 2015). In contrast, podoplanin expressing CAFs in breast carcinomas did not promote cancer cell invasion, but promoted angiogenesis (Suchanski et al., 2017)
Podoplanin as a promotor of tumor cell migration and EMT
In vitro, podoplanin is expressed on cell-surface protrusions, microvilli, filopodia and ruffles, and is involved in cell motility and migration by recruiting ezrin and promoting the rearrangement of the actin cytoskeleton (Martin-Villar et al., 2005; Scholl et al., 1999). In SCCs, podoplanin often co-localizes at the invasive front with E-cadherin (Wicki et al., 2006). However, in some oral SCCs, podoplanin expression is associated with downregulation of E-cadherin (Inoue et al., 2012a), suggesting that podoplanin expression in vivo promotes weakening of cell-cell contacts. This was demonstrated in experiments with immortalized HaCaT keratinocytes and mouse premalignant MCA3D keratinocytes, where forced podoplanin overexpression induced full EMT with loss of epithelial markers such as E-cadherin and keratins, and upregulation of mesenchymal proteins such as N-cadherin and vimentin (Martin-Villar et al., 2006; Scholl et al., 1999). EMT at the invasive front is the first step of the metastatic cascade, allowing tumor cells to disseminate to other tissues (Brabletz et al., 2018). Indeed, podoplanin expressing cancer cells exhibit increased migratory capacity in vitro and enhanced lymph node metastasis in vivo (Cueni et al., 2010;
Scholl et al., 1999). Podoplanin-induced EMT is mediated via the cytoplasmic tail binding to the ERM proteins and activation of the RhoA GTPase-ROCK signaling pathway, and association with membrane lipid rafts (Fernandez-Munoz et al., 2011;
Martin-Villar et al., 2006). The localization of podoplanin in the lipid rafts is mediated by the GXXXG motif in the transmembrane domain (Fernandez-Munoz et al., 2011).
The motif is highly conserved and was positively selected during evolution, suggesting its functional relevance (Renart et al., 2018). Podoplanin was also reported to have an ERM-independent interaction with CD44 at the leading edge of SCC cells, promoting directional migration of carcinoma cells (Martin-Villar et al., 2010).
In addition to promotion of single cell migration and EMT, podoplanin was also reported to promote collective cell migration and invasion in vivo and in vitro. In a mouse transgenic pancreatic cancer model, podoplanin was reported to promote tumor invasion without downregulation of E-cadherin (Wicki et al., 2006). Additionally, podoplanin was reported to promote collective cell migration and invasion in oral SCCs via downregulation of RhoA and upregulation of Cdc42 GTPase (Li et al., 2015).
Overall, is appears that podoplanin signaling and function in cancer cell invasion is cell- and context-specific. Furthermore, it should be noted that EMT is not an all-or- nothing process and intermediate EMT states exist that downregulate cell-cell contacts without downregulation of the associated markers (Brabletz et al., 2018).
Podoplanin in cancer cell invasion and ECM remodeling
Podoplanin expression is often found to correlate and co-localize with MMPs in invading tumors (Li et al., 2015; Mashhadiabbas et al., 2012; Tsuneki et al., 2012).
Furthermore, podoplanin can upregulate MT1-MMP via activation of small GTPase Cdc42 and stimulate oral SCC cell invasion (Li et al., 2015). Podoplanin and MT1- MMP form complexes and co-localize at the leading edge of migrating cells (Li et al., 2015). Podoplanin was also reported to stimulate TGF-β secretion in oral SCC cells, activating surrounding fibroblasts to upregulate MMP2 and MMP14 expression (Li et al., 2018). Activated CAFs then induce podoplanin expression in the cancer cells through the TGF-β–Smad pathway and enhance cell invasion by activating EGFR, AKT, and ERK signaling (Suzuki et al., 2008).
Cancer cell invasion and metastasis require degradation and remodeling of the ECM, including both the basement membrane and the interstitial matrix. The basement membrane is a specialized type of ECM underlying the epithelial and endothelial cells, providing a barrier to the neighboring tissues, a substrate for cell adhesion, and a platform for biochemical and mechanical signaling between the epithelium/endothelium and the external environment (Sekiguchi and Yamada, 2018).
Primary tumors form actin-rich membrane protrusions with focalized proteolytic activity, called invadopodia. These invadopodia degrade the ECM and perforate the basement membrane, enabling local invasion (Hoshino et al., 2013a). Typically, these invadopodia consist of actin dynamics regulators, scaffolding proteins, integrins and MMPs (Hoshino et al., 2013a). Podoplanin was reported to be a novel component of invadopodia in oral and breast cancer SCCs (Hwang et al., 2012; Martin-Villar et al., 2015). Recruitment of podoplanin to invadopodia requires binding to the ERM
proteins and association with lipid rafts (Martin-Villar et al., 2015). Podoplanin promotes invadopodia maturation and stabilization, but not formation, by activating the RhoC–ROCK–LIM kinase–cofilin signaling pathway promoting ECM degradation (Martin-Villar et al., 2015). Additionally, invadopodia formation and degradative activity were suggested to be dependent upon and enhanced by exosome secretion (Hoshino et al., 2013b). Thus, podoplanin was found to be both an exosome component and regulator of exosome secretion (Carrasco-Ramirez et al., 2016).
Podoplanin in pathological lymphangiogenesis and angiogenesis
Tumoral and peritumoral lymphangiogenesis is a hallmark of tumors and the first step towards metastasis (Karaman and Detmar, 2014; Stacker et al., 2014). Lymph node lymphangiogenesis in tumor draining lymph nodes is often detected even before cancer cells arrive, and is believed to facilitate cancer cell dissemination to distant organs and act as a niche for the survival of metastatic cells (Karaman and Detmar, 2014). The role of podoplanin in lymphangiogenesis is disputed in the literature.
Forced expression of podoplanin in a human breast carcinoma xenograft model promoted lymphangiogenesis and metastasis to regional lymph nodes without affecting primary tumor growth (Cueni et al., 2010). Additionally, several other reports have linked podoplanin expression in CAFs of breast, prostate, bladder, pancreas and ovary carcinomas with tumoral lymphangiogenesis and lymph node metastasis (Kitano et al., 2010; Pula et al., 2013). The exact molecular mechanisms by which podoplanin promotes lymphangiogenesis remain unknown, but secretion of pro- lymphangiogenic growth factors or cytokines, such as VEGF-C, VEGF-D and CCL21, has been suggested (Astarita et al., 2012). Additionally, galectin-8-, endothelin-1-, vilin-1- and tenascin-C-podoplanin interactions have been suggested to induce tumor lymphangiogenesis and metastasis (Cueni et al., 2010). In contrast, a lung squamous cell cancer cell line expressing podoplanin was reported to downregulate VEGF-C signaling and attenuate lymphangiogenesis and lymph node metastasis (Suzuki et al., 2010). Tumor cells and CAFs can also secrete extracellular vesicles (microparticles, microvesicles, exosomes) into the tumor microenvironment, which can promote tumor progression (Xu et al., 2018). Podoplanin was reported to be a