Reegulati
der Ma
ion and
I
Erl themati d
au
d cellul
Inaugur
langung sch-Nat der Univ
vor Karthic us Chid
K
lar role
ral-Diss
zur g des Do
turwisse versität
rgelegt v c Swam
ambaram
Köln, 201
es of co
sertatio
oktorgra enschaft zu Köln
von minathan
m, Indie
3
oronin
n
des
tlichen F n
en
protei
Fakultät
ins
t
Berichterstatter: Prof. Dr. Angelika A. Noegel Prof. Dr. Martin Hülskamp
Tag der mündlichen Prüfung: 15.04.2013
The present research work was carried out from Jan 2010 to Jan 2013 at the Centre for Biochemistry, Institute of Biochemistry I, Medical Faculty, University of Cologne, Cologne, Germany, under the supervision of Prof. Dr. Angelika A. Noegel.
Die vorliegende Arbeit wurde in der Zeit von Jan 2010 bis Jan 2013 unter der Anleitung von Prof. Dr. Angelika A.Noegel am Biochemischen Institut I der Medizinischen Fakultät der Universität zu Köln angefertigt.
giving me an opportunity to work in her lab, tireless help with this thesis and for her constant encouragements.
I convey my sincere thanks to Prof. Dr. Ludwig Eichinger for introducing me to Dicty and nurturing my interests in it.
I also would like to thank our collaborators especially Dr. Annette Müller-Taubenberger (LMU, München) and Dr. Jan Faix (MH-Hannover) for providing reagents for this study. I also would like to extend my thanks to Dr. Francisco Rivero (Hull York Medical School, UK) for his critical comments and constructive ideas during the course of the study. I specially thank to my previous mentors Prof. G. Shanmugam and Prof. Mariappan for their constant support.
I also thank Ms. Dörte Püsche for her help and cooperation with the administrative work that made my life easy. I take this opportunity to thank Gudrun and Budi for their help and for all the chocolates during my stay in the exile room.
My special thanks to Vivek who guided me through a lot of trouble since the day one of my stay in Cologne. I thank to Xin and Liu for showing me around Cologne in my initial days and for Sandra for her help in buying bike which I happened to ride mercilessly.
Very special thanks to Ms. Rosie who helped me a lot with my experiments and also for creating a friendly environment with more fun. I also thank Bhagyashri and Tanja for their help during microscopy sessions. I thank Mr. Rolf Müller for his good technical support in cloning. I also thank Mr. Berthold Gassen for all the wunderbar antibodies. I extend my special thanks to Sonja for her help with all the important reagents and media.
I also extend my special thanks to Napoleon (Neppo) and Salil for making sure I go to mensa and get to eat every day.
I also thank all members of the lab, Atul, Kosmos, Ilknur, Ping, Pranav, Szeman, Ramesh, Jan, Khalid, Lin, Qiuhong, Natalia and Christoph, Kurchi, Raphael, Anja, Kalle, Juliane, Maria, Martina, Sacha, Sajid, and Sandra for their good company, help and support. I also thank my cricket mates Ram, Ganapathy, Sabari, and Vinoth.
My special thanks to Taran, Aarna, Sila, Harmony, Inbha, and Vasundhanay for bringing new life to my project.
I express my heartfelt gratitude to my brother Guru who introduced me to the molecular biology field, my parents and family members for their patience, and encouragement throughout my life.
Finally I would like to thank Harry Potter and Honor Harrington, though they don’t exist! for their company in difficult times.
Table of Contents
1.Introduction………..1
1.1 Coronin proteins – structure and functions………1
1.2 Rho GTPases………...2
1.3 CRIB effector proteins………...4
1.4 CRIB effector proteins in D. discoideum………...6
1.5 PAK kinases – structure and functions………...7
1.6 Myosin II regulation in D. discoideum………...8
1.7 D. discoideum family of PAK kinases………...9
1.8 Aim of the study………...10
2. Results………...11
2.1.1 Sequence and structure of the coronin CRIB motif………...11
2.1.2 Structural analysis of the coronin CRIB domain………...12
2.2.1 Expression and purification of Dictyostelium coronin GST-fusion constructs...14
2.2.2 Interaction of the Coronin CRIB motif with Rac GTPases………...16
2.2.3 Direct interaction of the Coronin CRIB motif with Rac GTPases………...18
2.2.4 Subcellular localization and dynamics of the coronin CRIB domain………...19
2.3 Interaction of Dictyostelium coronins with Rac GTPases………...20
2.4.1 Coronin regulates myosin II function………...25
2.4.2 D. discoideum coronin interacts with Rac GTPases that regulate myosin II function………...27
2.5 Expression and characterization of coronin carrying a mutated CRIB motif...28
2.5.1 Coronin CRIB mutant constructs and expression………...28
2.5.2 Cellular localization of coronin CRIB mutant………...31
2.5.3 The coronin CRIB mutant retains the biochemical properties of wild-type coronin………...32
2.5.4 Expression of Coronin CRIB mutant rescues multi-nuclearity but fails to rescue the myosin II phenotype of coronin knock-out cells...33
2.6 Expression and characterization of a dominant negative PAK in corA- cells...36
2.6.1 corA-cells show elevated Rac-GTP levels………...38
2.6.2 D. discoideum coronin interacts with PAKa………...39
3. Discussion………...42
4. Materials and Methods………...50
4.1 Materials………...50
4.1.1 Oligonucleotide Primers………...50
4.1.2 Primary antibodies………...50
4.1.3 Strains used in the study………...51
4.2 Methods………...51
4.2.1 Growth of Dictyostelium strains………...51
4.2.2 Cloning, expression and purification of GST and GFP fusionproteins…...52
4.2.3 Loading of Rac GTPases with GDP or GTPS………...52
4.2.4 Immunoprecipitation and pull down experiments………...53
4.2.5 Mutant analysis………...53
4.2.6 Miscellaneous methods………...54
5. Summary………...55
6. References………...57
7. Abbreviations………...67
8. Erklärung………...68
9. Lebenslauf………...69
1. Intr
1.1 Co The dy physiol morpho the ma protein propelle all majo into tw domain a uniqu at thei contain contain WASP/
Figure of the domain Coronin the Arp shown
roduction
ronin prot ynamic reg
ogical pr ogenesis ( ajor regula s and con er structur or taxa of wo groups n(Figure 1) ue domain r C-termin na tandem n a highly /SCAR fam
1: Structu beta-pro n is show ns function p2/3 compl
to bind F
n
teins - stru gulation o rocesses
Lee and D ators of cy ntain sever re. Coronin
the eukar s: short a ). Short co
of varying nus that is
beta-prop acidic dom mily membe
ural topolo opeller do
n in grey ( n in modu
lex andall F-actin. Co
ucture and f the cyto
like cell Dominguez ytoskeleton ral copies n proteins ryotic kingd
and long oronins con
length. In s required peller dom main in the ers (Uetrec
ogy of the omains ar
(taken fro lating actin
short coro oronins loc
d function oskeleton o
migratio z, 2010). T n. Coronins
of WD rep are highly dom exce coronins ntain a sing
addition, t d for homo main and la eir extreme
cht and Be
e two maj re colored
m McArdl n dynamic onin homol calize to th
ns
of a cell f n, vesicle he coronin s belong t peats that y conserve
pt plants.
, dependi gle beta-p they conta o-oligomer ackthe coi e C-termin ear, 2006).
or classes d and nu le and Hof s by bindi ogues inve he anterior
forms the e trafficki n family of to the WD form a se d proteins They are ing on th ropeller do in a short c rization. Th
iled-coil do us that ha
s of coron umbered.
fmann, 20 ng to filam estigated to
r regions o
Intro
basis of ing, and
proteins is D repeat fa even blade
and are f broadly cl he beta-p omain follo coiled-coil he long c omain. Th as similarity
nins. The The coile 08).
mentous ac to date hav of migratin
oduction
1 several tissue s one of amily of ed beta-
found in assified propeller owed by domain coronins ey also y to the
blades ed coil
ctin and ve been ng cells
2
where they regulate the actin dynamics in the cortex (Cai et al., 2007). The single coronin in yeast binds to F-actin and microtubules in vitro and regulates dynamic actin structures such as actin patchesin vivo (Goode et al., 1999). In Drosophila, mutations in coronin disrupt the actin cytoskeleton of the embryonic imaginal disks indicating that it is essential for morphogenesis (Bharathi et al., 2004). Mammalian Coronin 7 localizes to the Golgi network and plays a role in Golgi trafficking (Rybakin et al., 2004; 2006)
The Dictyosteliumdiscoideum genome encodes two coronin homologues, a short coronin encoded by the corA gene with a conserved coiled coil domain and a longer homologue with a tandem beta-propeller domain, CRN7 encoded by the corB gene.
D.discoideumcoroninis thefounding member of the coronins and was identified as a co-purifying protein from acto-myosin complexes and has been found enriched in crown like projections in the dorsal surface of the cells (de Hostos et al., 1991).
deHostos et al.(1993) later created a coronin deficient cell line which showed several interesting phenotypes implicating the protein in physiological processes like cytokinesis, migration, cell polarity, and morphogenesis.CRN7, aDictyostelium homologue of CaenorhabditiselegansPOD-1 has been implicated in actin driven processes and Legionella pneumophila internalization (Shina et al., 2010). Deletion of both coronin homologues in D.discoideumhighlightedthe factthat even though they are involved in the same cellular processes, they act antagonistically to each other (Shina et al., 2011).
1.2 Rho GTPases
Rho-like proteins are small, monomeric GTPases of the Ras superfamily. They act as a molecular switch in the cell by cycling between an active (GTP-bound) and an inactive (GDP-bound) conformation. This activation cycle is regulated by two classes of enzymes, GEF and GAP proteins. GEFs or GuanosineExchange Factors activate the Rho GTPases by exchanging GDP for GTP, and GAP or GTPase Activating Proteins increase the intrinsic GTP hydrolysis of Rho GTPases thereby effectively terminating the signal by converting them into an inactive form (GDP-bound). A third class of regulatory proteins known as GDI (GDP Dissociation Inhibitors) regulates Rho GTPases by binding to the GDP bound (inactive) form of GTPases and prevent their spontaneous activation (Figure 2). They also bind to the isoprenyl moiety of Rho
GTPas cytosol Once a molecu in seve morpho signific regulat have b sub-fam stress f like lam
Figure bound and GA motif o to mem The D.
true ho studied
ses through and mem activated, ules and tra eral cellular ogenesis,
ant aspect ion of the een descr mily were s
fibres form mellipodia a
2: Rho ) and act APs inact of GTPase mbrane(ta
. discoideu omologue o d in detail
h an immu brane.
the Rho G ansduce th r processe
and cytok ts of Rho G
actin cyto ribed so fa
studied in mation,Rac and filopod
GTPase ive forms ivates the es is show
ken from um genom
of Rho or (Rivero e
unoglobulin
GTPases c he signal to es like cell
kinesis (Et GTPases i oskeleton
r. Of them detail. Ma and Cdc4 dia, respec
cycle. Rh s (GTP-bo em by incr wn in cros Etienne-M me encodes
Cdc42 is et al., 200
n-like fold
can bind t o downstre
polarity, g tienn-Mann is their abi
in the cel m,several m
ammalian R 2 regulate ctively (Hal
ho GTPas ound). GE
reasing G ssed line t Manevillea
s nearly 1 absent. S 1, Vlahou
and regula
o several eam event ene transc neville and ility to link
l. Twenty members o RhoA regu the forma l, 1998).
ses cycle Fs catalyz TP hydrol through w and Hall, 2
8 Rac rel ome of the and River
ate their c
proteins k s. Rho GT cription, ve d Hall, 20
membrane mammalia of the Rho, ulates myos
tion of acti
between ze the ex lysis rate.
which Rho 002.
ated GTPa e Rac GTP o, 2006).
Intro
cycle betwe
known as TPases par esicular tra
002). One e receptor an Rho G , Rac, and sin assem in rich prot
n inactive xchange re . The pren o GTPases
ases, how Pases hav The Rac1
oduction
3 een the
effector rticipate fficking, of the rs to the
TPases d Cdc42 mbly and trusions
(GDP- eaction nylation
s binds
wever, a ve been 1 family
4
consists of three members (Rac1A, 1B, 1C) and is considered as orthologues of mammalian Rac1. Rac1A, 1B, and 1C regulatethe actin cytoskeleton and cell motility (Faix et al., 1998). In addition, Rac1A and RacE are required for cytokinesis (Larochelle et al., 1997). RacB is required for chemotaxis and morphogenesis (Park et al., 2004), while RacC is implicated in phagocytosis and is regulated by phosphatidylinositol 3-kinaseactivation (Han et al., 2006). RacG is required for the regulation of cell shape, motility, and phagocytosis (Somesh et al., 2006a). RacH has been implicated in vesicular trafficking and intracellular immunity to Mycobacterium (Somesh et al., 2006b). Furthermore, the D. discoideum genome encodes numerous exchange factors (RacGEFs) and GAPs (RacGAPs) and the function of handful of them are known (Park et al., 2004, Faix et al., 1996). In addition, there are two GDI homologues present and deletion of either one or both of them results in a defective cytokinesis and contractile system (Rivero et al., 2002).
1.3 CRIB effector proteins
Rho GTPases bind to several downstream target molecules known as effector proteins. These proteins specifically interact with the GTP-bound form of Rho GTPases and this is achieved by recognizing conformational changes in the ‘effector region’ (switch I) of Rho GTPases (Bishop and Hall, 2000). Most of the Rac and Cdc42 effector proteins, if not all, contain a conserved CRIB motif (Cdc42 and Rac – interactive binding). The CRIB motif is the minimal effector binding region for Cdc42 and Rac and is 15 amino acids long with the consensus ISXPXXXXFXHXXHVG. This small motif forms part of a larger binding region (also known as PBD-p21-binding domain or GBD-GTPase binding domain) that has been shown to be required for GTPase interaction. The CRIB motif was first reported by Burbelo et al. in 1993.
Subsequently, Hall and co-workers identified a host of candidate effectors proteins on the basis of homology searches to the CRIB region (Burbelo et al., 1995). The repertoire of effector proteins expanded phenomenally in the last decade or so and includes protein families having diverse sets of structure and function. Some exemplary families are kinases (ser/thr protein kinase and tyrosine kinase), actin- binding proteins, and scaffold proteins (Bishop and Hall, 2000).
The GBD of WASP (Wiscott Aldrich syndrome protein) and ACK (Cdc42 and Rac- interactive kinase) were the first reported crystallographic structures bound to GTPase(Abdul-Manan et al., and 1999, Mott et al., 1999). Subsequent mutational
Introduction
5
studies of the CRIB motif provided a significant insight into the binding interface between GBD and GTPases. The interaction is mediated by both polar and hydrophobic contacts involving highly conserved residues in the CRIB motif. The conserved isoleucine, serine, and proline in the N terminus of the CRIB motif form hydrophobic contacts with the 5 helix of the GTPase. The conserved Asp38 in the switch I region of Cdc42 and Rac1 interacts with one of the conserved histidines (HXXH) in the CRIB motif. Mutation of either of these histidines greatly affects the interaction of CRIB effector with GTPases. The adjacent residues of the CRIB motif show less conservation among CRIB effector proteins. However, these residues make extensive contacts with the switch I and switch II regions of the GTPases and appear to be important in response to the nucleotide switch (Morreale et al., 2000).
The CRIB motif containing effector proteins bind preferentially to GTP-bound Rho GTPases and connect the activation of GTPases to a broad range of downstream responses. Once bound to the active GTPases, the activity of the CRIB effector proteins might be regulated in several ways such as activation, sub-cellular localization and others. The most common mechanism of effector activation by Rho GTPases appears to be the disruption of intramolecular auto-inhibitory interactions, which exposes functional domains within the effector protein. For example, the two related CRIB domain containing effector proteins WASP and N-WASP are regulated by intramolecular inhibition. These proteins share similar domain architecture and are involved in the relay of signals from cell membrane receptors to the actin cytoskeleton. The WASP protein contains an N-terminal GBD (GTPase binding domain) required for Cdc42 interaction, and a C-terminal VCA domain (Veprolin homology, Cofilin homology, acidic region segment) involved in Arp2/3 complex mediated actin polymerization. In an auto-inhibited state, the cofilin homology domain forms an intramolecular auto-inhibitory interaction with the N-terminal GTPase binding domain and effectively masks the VCA domain. Cdc42-GTP activates WASP by binding to the GBD and subsequently relieving the auto-inhibitory intra-molecular interactions. The relieved VCA domain then recruits Arp2/3 complex and initiates actin polymerization (Rohatgi et al., 2000). A similar mechanism of activation has also been observed in other Rho GTPase target proteins like PAK (kinase activity), mDia (actin polymerization) and several others (Hoffman and Cerione, 2000). A different mode of activation has been observed in IQGAP protein, which is involved in
6
actin cross-linking function where Cdc42-GTP was shown to regulate the cross- linking activity of IQGAP in vitro (Fukata et al., 1997).
In recent years, proteins with less conserved CRIB motifs have also been shown to interact with Rho GTPases in a GTP dependent manner. For example, POSH2 is an E3 ligase and scaffold protein that interacts with Cdc42-GTP through a partially conserved CRIB motif (conserved ISxP sequence) (Kärkkäinen et al., 2010).
Similarly, phospholipase D2 (PLD2) contains two weakly conserved CRIB motifs in its PH domain through which it interacts with Rac2 (Peng et al., 2011). In some cases, proteins with a partial CRIB sequence use its adjacent structural modules for GTPase interaction. Par6 in Drosophila has been shown to be required for neuroblast cell polarity and asymmetric cell division. It contains a PDZ domain downstream to a partial CRIB motif. Both domains are required for Cdc42-GTP interaction (Garrard et al., 2003;Joberty et al., 2000). PlexinB1, a functional semaphorin receptor has been reported to contain a partial CRIB sequence embedded in its cytoplasmic domain.
Deletion analysis showed that the plexin-Rac binding domain is significantly larger than the CRIB motif (Haris et al., 2000).
1.4 CRIB effector proteins in D. discoideum
In D. discoideum, several Rac effector proteins have been characterized in detail.
DGAP1 and GAPA are two IQGAP-related proteins that regulate cytokinesis in D.discoideum. Both proteins interact with Rac1a through a conserved GRD (GTPase related domain) domain. The actin binding proteins cortexillin I and II form a quaternary complex with DGAP1 or GAPA and activated Rac1a (Faix et al., 1998;Mondal et al., 2010). Filamin, an actin cross-linking protein has been shown to interact with activated Rac1a and associates with cortexillin I (Mondal et al., 2010).
Formin homology proteins regulate cytoskeletal remodeling during cytokinesis, cell polarity, and development. The diaphanous or Dia-related FH proteins (DRFs) constitute a subclass of FH related proteins and a D.discoideumformin homologue, mDia2, has been shown to localize to filopodia tips and regulate actin polymerization.
mDia2 binds to activated Rac1a and is required for filopodia formation (Schirenbeck et al., 2005). RacC interacts with WASP and stimulates F-actin polymerization via the activation of WASP. RacE is requited for 14-3-3 localization to the cortex and 14-3-3 mediated myosin II assembly (Robinson DN, 2010). However, no direct RacEinteraction partner has beenreported so far. RacB specifically interacts with
Pakaan through 1.5 Pak PAK o GTPas catalyti for Rac that co inhibito basal k comple as a h regulat domain binding and su (Figure phosph Bokoch
Figure is sequ
nd Pakc in h PAKs act kkinases –
r p21-activ ses. They c
c domain.
c or Cdc42 ontributes ory segme kinase act ex of heter
homodime ory domai n of the oth g of Rac or
bsequent e 3). PAKs
horylation o h, 2003).
3: PAK ki uestered i
its activate tivation (fo – structur vated Kina contain an
The N-ter 2 binding.
to overall nt in its C tivity. Add rotrimeric G
r in the c n of one P her with the r Cdc42-GT trans-auto s have be of several c
inase activ in the dim
ed form an or details se
e and func ases are o
N-termina rminal dom
This motif binding a C-terminus
itionally, a G proteins cells in a PAK1 mole e PDB/CR TP to the C o phosphor
een shown cytoskeleta
vation. PA mer interfa
nd regulate ee next ch ctions one of the al regulator main harbo
f forms a p affinity. Th s known a
a conserve exists at a trans-inh ecule bind IB region f CRIB doma
rylation at n to regul al proteins
AK exists ce. Bindin
es myosin apter).
e major do ry domain ors the CR part of a m he PBD a
s ‘inhibito ed binding
the extrem hibited con s and inhi forming the
ain relieves many site late cell s s like myos
as a dim ng of Cdc4
II assembl
ownstream and a C-te RIB motif th
more inclus lso overla ry switch’
g site for me C termi
nformation bits the C- e dimerizat
s this inhib s leads to shape and
in (Zhao a
er and the 42-GTP re
Intro
ly and che
effectors erminal con
hat is resp sive doma
ps with a that contr the G inus. PAK . The N-t -terminal c tion interfa bited confo kinase ac d polarity and Manse
e kinase d elieves thi
oduction
7 motaxis
of Rho nserved ponsible in,PBD, n auto- rols the
subunit 1 exists terminal catalytic ace. The ormation ctivation through r, 2012;
domain is auto-
inhibit orange 1.6 My Myosin myosin chain ( assemb phosph mediate D.disco deletion cytokin During and sh speed D. disc filamen residue phosph rescue phosph de la R
ion and a e and red
osin II reg n II is a con n heavy ch (RLC). A u ble into bip horylation
ed regulat oideum, m n of mhcA
esis in su chemotax howed a ce
due to the coideum c nts is nega es in its homimetic
the mhcA horylation i Roche et al
activates (taken fro gulation in
nventional, hain (MHC universal p polar filame
of the ligh tion of ce myosin II h
Aled to a uspension xis, mhcA-c
ell polarity eir inability can self-as
atively reg heavy c mutant (3X A- defects
s required ., 2002).
PAK. The m Hoffma nD.discoid
, two head C), an ess property of
ents. Filam ht and hea
llular proc heavy cha a plethora
and were cells were y defect. A to retract ssemble in gulated by chain (Stit
XASP) nor s demonst
for myosin
e CRIB m an and Cer
deum
ded myosin ential light f myosin I ment assem
avy chains cesses like ain is enco of defect e unable to
unable to Additionally the uropod nto bipola
phosphor tes et al
r a non-ph trating the n II functio
motif and rione, 200
n that cons t chain (E I is the ab mbly and d
s and form e cytokine
oded by a ts. mhcA- o develop
suppress l y, mhcA- c d (Titus et ar filament
rylation of ., 1998).
hosphorylat dynamic ons (Bosgra
Cdc42 ar 00).
sists of two LC), and bility to sp dis-assemb ms the ba sis and c a single g
cells sho beyond t ateral pse ells showe
al., 1993) s. The fo three con
Expressio table muta
regulation aaf and va
re highligh
o copies ea a regulato pontaneous bly is regul asis for m cell movem
gene (mhc owed a d the mound eudopod fo ed a reduc ). The myo ormation o nserved th on of ne ant (3XALA n of heavy an Haaster
8 hted in
ach of a ory light
sly self- lated by
yosin II ment. In cA) and efective d stage.
ormation ced cell osin II in of these
reonine either a
A) could y chain rt, 2006,
Introduction
9
Figure 4: Myosin II regulation in D. discoideum(taken from de la Roche et al., 2002).
D. discoideum encodes four myosin heavy chain kinases, MHCKA through MHCKD, which are responsible phosphorylation of the three threonine residues in the MHC tail region that are critical for filament formation (Luo et al., 2001). They belong to the alpha-kinases family and apart from a conserved catalytic domain; they possess a WD repeat domain that is required for substrate targeting. Each MHCKs displays different cellular localization and was suggested to have separated functions in the cell. MHCKA is primarily localized in the anterior F-actin rich regions where it enables formation of new pseudopods by disrupting myosin filaments. MHCKC localization is dependent on myosin II and has been suggested to regulate myosin II during uropod retraction and cleavage furrow formation. MHCKB is localized in the cytosol and may serve a role in maintaining basal MHC phosphorylation levels (de La Roche et al., 2002). The molecular mechanisms underlying activation and localization of MHCKA have been studied in detail. MHCKA has been shown to be activated primarily by auto-phosphorylation and recently, it was shown that F-actin can activate MHCKA through increasing its auto-phosphorylation rate. MHCKA contains a coiled-coil domain through which it binds to F-actin and localizes to anterior regions (Steimle et al., 2001).However, it was suggested that the kinase activity one or more MHCKs was negatively regulated by upstream PAK kinases during cell migration: particularly in the posterior regions of migrating cells (Chung and Firtel, 1999).
1.7D. discoideum family of PAK kinases
The D. discoideum PAK kinase family consists of three members, PAKa, PAKb, and PAKc, with a conserved CRIB motif in the N-terminal regulatory region and a catalytic domain in its C-terminus. PAKa has been shown to localize to the posterior regions of migrating cells where myosin II is enriched (Chung and Firtel, 1999). Cells deficient in PAKa have been shown to exhibit altered F-actin cytoskeleton, defective cytokinesis and cell polarity. PAKa tagged with a membrane targeting signal was used to show that PAKa activates actin polymerization at the cortex. Most strikingly, PAKais required for myosin II assembly and it regulates myosin assembly by inhibiting one or more myosin heavy chain kinases (MHCKs), however it did not phosphorylate myosin directly. A dominant negative PAKa containing the CRIB and the catalytic domain has been shown to inhibit phagocytosis by Müller-Taubenberger et al. (2002). In
10
addition,this group showed that PAKa can interact with Rac1a specifically in its GTP- bound form, and that a small region in the N-terminus is required for PAK localization to the cortex. PAKb was shown as a heavy chain kinase for unconventional myosin I and is involved in the formation of phagosomes (de la Roche et al., 2005). PAKcis a PH domain containing member and localizes to the plasma membrane upon chemo- attractant stimulation. PAKc kinase activity increased upon cAMP stimulation and was activated specifically by RacB. A PAKc carrying a mutation in the CRIB motif exhibited higher basal kinase activity highlighting the role of Rac GTPases in PAK regulation (Lee et al., 2004). However, PAKccould also regulate myosin assembly.
However,PAKa has been suggested to be the major player in regulation of myosin dynamics. Accordingly, Chung and Firtel(1999) suggested that PAKa localizes to the rear of the moving cells and enhances myosin assembly by inhibiting the activity of heavy chain kinases. PAKain turn is activated by phosphorylation by Akt and Rho GTPases.
1.8. Aim of this study
The aim of this work is to study the interaction specificity of coronins and Rho GTPases in the model system D. discoideum and
1. to analyze and characterize the putative CRIB domain of D. discoideum coronin proteins,
2. to understand the significance of Coronin-Rac interaction in the physiological processes of the cell,
3. to elucidate the molecular signaling pathway in which coronin and Rho GTPases are involved.
.
2. Res
2.1.1 S Coronin regulat coronin propelle conserv and bin the D.
compa domain
Figure region shown similar D.disco acids 1 amino a helix of motif in specific in one absent
sults
Sequence n proteins
e the actin n proteins c
er domain ved amino nd Rho GT . discoide
red the C n sequence
5: Sequ s of sele n on the t r amino ac oideum sho
117 and 1 acids and f Rho GTP nteracts w city (Abdul of the con (Figure 5)
and struc belong to n cytoskele
contain a C n (Xavier e o acids seq
TPases (B eum coron
RIB doma es and fou
ence alig cted CRIB op. Highly cids are bo ort coronin 133. The
these ami Pases. The with the sw
-Manan et nserved h ).
ture of the the family eton of the
CRIB like m et al., 200 quence tha Burbelo et
nin seque ain of D. d
nd a high d
nment of B-containi y conserv oxed yello n contains N-terminal no acids a e two cons witch I regi
al., 1999) istidine res
e coronin y of WD re e cell. Rece
motif betw 08). The C at enables
al., 1993).
ence using discoideum degree of c
f the CRI ing protei ved seque ow.
a highly c l half of th are known t
served his on of Rho . In D.disc sidues and
CRIB mot epeat dom ently, it wa een blade CRIB dom
the protein We have g sequenc m coronin
conservati
B domain ins. The c ences are
conserved he CRIB to form ext tidines in t o GTPase oideumco d the phen
tif
main contai as reported
2 and blad ain is a s ns containi identified ce alignm proteins w on (Figure
n in coro consensu shown in
CRIB mot motif cont tensive co the C-term
and deter oronin, ther nylalanine
ning prote d that mam
de 3 of the small stretc
ing it to re a similar ment. We
with know e 5).
onin with us CRIB m
n red box
tif between tains hydro ntacts with minus of th
rmines nu re is a sub (F) is com
Results
11 eins and mmalian
eir beta- ch of a cognize
motif in further n CRIB
similar motif is xes and
n amino ophobic h the 5 e CRIB cleotide stitution mpletely
D. disc beta-pr each o compa and ma
Figure CRN7 The co showed share a termina 2.1.2 S Sequen structu CRIB m the cor the cor To inve model atoms model (Figure Deviati
coideum en ropeller do of its prop
rison. We ammalian P
6: Seque homologu omparison
d a somew a conserve al half is ve Structural
nce alignm ral elemen motif is in a ronin CRIB ronin1A (2A
estigate ho and know and analy are in goo e 7.1). To on) values
ncodes als main (Shin peller dom
have also POD homo
ence align ues with s of the CR what lesser
ed N-term ery weak.
analysis o ment of th nts of the a surface a B domain,
AQ5) struc ow well the wn crystal s
yzed. The od agreeme
o assess s between
o a homolo na et al 20 mains and
included t ologue CR
nment of t imilar am RIB doma r conservat
inal half o
of the coro he D. disc recently c accessible
we modele cture as a t
e modeled structures
overall ar ent with th the mode superimpo
ogue of the 10). We re
aligned t the CRIB RN7 (Figure
the CRIB d ino acids ain sequen tion of the of the CRIB
onin CRIB coideum c crystallized loop. To g ed the stru template (A d structure
(2AQ5) w rangemen e correspo el accurac osed mode
e long coro etrieved the hem with sequence e 7).
domain of boxed ye nce of CR critical am B domain,
B domain coronin pr d coronin1 gain further ucture of D Appleton e
matches were super ts of the s onding elem cy, the RM el and crys
onins, CRN e CRIB seq known C from hum
f mamma llow.
N7 and m mino acids.
the conse
roteins wit A protein r insight in D. discoide t al., 2006 the X-ray imposed o secondary ments in th MSD (Roo tallograph
N7, with a quence pre CRIB doma an coronin
lian coron
mammalian While all p ervation in
th the sec showed t to the stru eum coroni
).
data, the on their ba structures he X-ray s
ot Mean ic structure
12 tandem esent in ains for n CRN2
nin and
n CRN2 proteins n the C-
condary that the
cture of in using
coronin ackbone s in the
tructure Square es were
calcula sugges the ho analysi good m Ramac model
Figure discoid templa known structu We hav of the C expose be invo unavail
ated. The sting an op mology m s and foun modeling q chandran p lies in the f
7.1: Stru deum cor ate. A sup n structure
ure is show ve used th CRIB doma ed while th
olved in h lable for in
RMSD va ptimal mod model, we nd that the quality. Fu plot and o favored re
uctural mo ronin stru perimpose e in blue wn.
he modeled ain. We fo
e C-termin hydrogen b teraction (
alue for t deling qual used PRO e Z-score c urthermore
bserved th gions of th
odeling of ucture wa ed image is shown
d coronin s und that th nal sequen bonding w Figure 7.2
the backb lity. To det OSA-Web, calculated , we used hat a high he plot (dat
f the D. d as modele
of the m n at the l
structure to he N-termin nces are e with neighb
).
bone atom termine the
an online by the an d the Swis her percen
ta not show
discoideum ed using odeled st eft. At th
o determin nal half of mbedded boring beta
ms was fo e stereo-c e tool for nalysis tool ss-PDB vie
tage of am wn).
m coronin coronin1 tructure in
e right th
ne the struc the CRIB d in the beta a sheets
ound to 0 hemical qu
protein st l agrees w ewer to v mino acids
n protein.
1A (2AQ5 n green a he final m
cture and domain is a-sheets a and there
Results
13 0.29 Ao
uality of tructural well with iew the s in the
The D.
5) as a and the modeled
position surface and may fore be
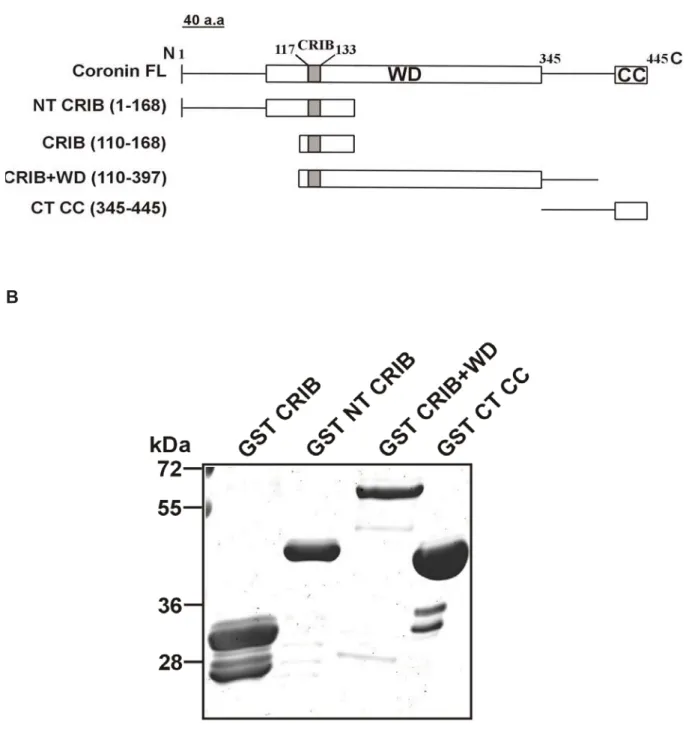
Figure structu (2AQ5) shown 2.2.1 constr To add differen represe 110-16 part of WD re without encodin pGEX were a mM IP polypep (Figure
7.2: Loca ure. The D ) as a tem n with the
Expressio ructs.
dress the b nt GST-fus enting the 68), a cons coronin (G peat doma t the CRIB ng the res 4T-2. The llowed to g PTG (isopr
ptides we e 8B).
ation of th D. discoid mplate. Bo
CRIB dom on and
biochemica sion constr last strand truct conta GST-CRIB ain (blades B motif (a spective re e DNA was grow until t ropyl -D-t re purified
e D. disco eum coro oth the top main highl
purificatio
al propertie ructs. The d of blade aining the C B-NT, amin
s 2-6, ami mino acid egions we s transfect the OD600 thio-galacto d using G
oideum co onin struct p view an ighted in on of D
es of Coro e construct 2 and par CRIB dom no acids 1
ino acids ds 345-445 ere cloned
ted into E 0 reached
oside). Th Glutathione
oronin CR ture was m nd the sid
red.
Dictyosteli
onin CRIB ts were: th rt of blade ain togeth
-168), a f 110-397) 5) (Figure
into the Escherichia 0.6-0.8 be he induced e-Sepharos
RIB domain modeled u e view of
um coro
domain, w he CRIB d
3 (GST-C er with the usion cons and the C
8A). The bacterial e a coli strain efore induc d cells we se beads
n in the m using cor f the struc
onin GST
we generat domain of CRIB, amin e entire N-t struct of th C terminal
DNA seq expression n XL1-blue cing them w ere lysed a
(GE Hea
14 modeled ronin1A cture is
T-fusion
ted four coronin no acids terminal he core
domain quences n vector
e. Cells with 0.5 and the althcare)
Results
15 A
B
Figure 8: A. Schematic diagram showing the different GST fusion polypeptides of coronin. The position of the amino acids is indicated. The modular structure of coronin with the location of the CRIB domain is shown above for comparison. B. The different GST fusion peptides were expressed in E.coli XL1-blue and purified as GST fusion products using Glutathione Sepharose beads. The polypeptides were separated on a 12 % polyacrylamide gel and stained with Coomassie Blue. On the left the position of molecular weight markers is indicated.
2.2.2 In To test discoid genom GTPas Dictyos Rac1a, Dictyos respect Glutath The co protein In our i CRIB d did not is in fac
nteraction t if the CR deum, we
e codes ne ses sub-fa
stelium Ra , a homolo stelium eq
tive GFP- hione-Seph
lumns wer s were pro in vitro pul domain cou
. This data ct a CRIB m
n of the Co RIB doma
carried ou early 18 R
mily of R ac family G
ogue of m quivalent o
-fusion pro harose col
re washed obed with G
ll down ex uld precipit a suggests
motif (Figu
oronin CRI in of coro ut an in vi ho-like GT Rho-like sm
GTPases a mammalian
of the ma oteins we
umns con several tim GFP mono
periments, tate GFP-R
that the m ure 9).
IB motif w onin can in vitro GST p TPases and mall GTPa
as GFP-fu Rac1 pro ammalian
re lysed ntaining di
mes after t oclonal anti , the coron Rac1a from minimal Ra
with Rac G nteract wit pull-down d almost a ases. We usion prote oteins, and Rho GTP and the fferent co two hours o
ibody K3-1 nin GST-fu m the cell l c1a GTPa
GTPases th Rho-like
assay. Th ll of them
expressed eins in AX d RacE wh Pase. Cells lysates w ronin GST of incubati
84-2.
sion peptid ysate while se binding
e GTPase he D. disc belong to t d several X2 wild typ
hich might s express were loade
T-fusion pe on and the
des harbo e the GST g region in
16 es of D.
coideum the Rac of the pe cells:
be the ing the ed onto eptides.
e bound
ring the T control coronin
Figure CRIB, expres specifi stainin We furt discoid GFP-R protein domain control
Figure uncoat The bo GST f weight
9: Glutat GST CRIB ssing GFP
ic mAb K ng.
ther tested deum such RacE were s bound to n of coroni
.
10: Gluta ted beads ound prot fusion pro
ts are indi
thione-Sep B+WD, an P-Rac1a. T K3-184-2.T
d if this sma as RacE o incubated o Glutathio n did inter
athione-Se s were inc tein was i
oteins we cated at t
pharose b nd GST C
The pull-d he GST f
all region c or RacC (s with equa one-Sepha act with R
epharose b cubated w mmunobl ere detect
he left.
beads coa CT CC wer down elu usion pro
could also see below) al amounts arose bead RacE. Some
beads coa with AX2
lotted with ted by P
ated with re incuba ate was oteins wer
interact wi ). Cell lysa s of GST a ds. As sho e binding w
ated eithe cell lysate h GFP an PonceauS
GST, GST ted with A immunob re detecte
th other R ates of AX2 and GST-C own in Fig
was obser
r with GST es expres
tibody mA staining.
T CRIB, G AX2 cell lotted wit ed by Pon
Rac GTPas 2 cells exp CRIB recom
ure 10, th rved with th
Tor GST-C ssing GFP AbK3-184 . The mo
Results
17 GST NT
lysates th GFP nceauS
es in D.
pressing mbinant e CRIB he GST
CRIB or P-RacE.
4-2. The olecular
2.2.3 D To det whethe active cleavag corresp amoun repeate These with Di quantif forms o the CR (GTP l loaded showed as com A
Direct inter ermine wh er coronin
(GTP bou ge and pur ponding to ts of GST ed washing
in vitro bi ictyostelium ied the am of Racs. U RIB domain
loaded). W with GTP d increase mpared to th
raction of hether the
interacts w nd) states rified the C
aa 1-168.
or GST-R g, the bou inding exp m Rac GT mount of re
pon plottin n of coron While near PS, only ed binding
he GDP-R
the Coron interactio with the R , we relea CRIB fragm
This purifi Rac GTPas
und protein periments s TPases Ra combinant ng the grap nin slightly
rly 44.7%
37% was with the C RacC (27%
nin CRIB m on of coro Rac GTPas ased the C ment with a ied polype ses preloa ns were an
showed th ac1a and t peptide b ph with the y preferred
of the inp s bound to CRIB fragm ) (Figure 1
motif with nin with R ses in the CRIB from an apparen
ptide was ded with e nalyzed by hat the cor RacC (Fig bound to ei
e input set the active put CRIB o GDP-Ra ment (30%
1B).
h Rac GTP Rac GTPas eir inactive GST-NT C nt molecula further inc either GDP
y Coomas ronin-CRIB gure 11A)
ther GDP at 100%, e form of fragment ac1a. Sim ) when loa
Pases ses is dire (GDP bo CRIB by th ar mass of cubated wit P or GTP
sie Blue s B motif int . Additiona
or GTPS we observ the Rac p bound to milarly, Rac
aded with
18 ect and ound) or hrombin f 18.000 th equal S. After staining.
teracted ally, we S loaded
ved that proteins Rac1a cC also
GTPS
B
Figure Throm Rac1a hour o gels an the lef plotted two ind These for the interact 2.2.4 S To furth the DN pBsrN2 transfe studied of GFP GFP-co
11: Dire bin cleave
and GST- of incubati nd stained ft in kDa.
d in a grap dependen
data sugg e activated tions (Burb Subcellula
her unders NA fragme 2 under th cted into d using imm P-cor-CRIB
or-CRIB w
ect intera ed NT CR -RacC tha ion, the bo d with Coo B. The bo ph with th nt experim gest that th d form of belo et al., r localizat stand the in ent coding he control
AX2 cells munofluore B during ra
was distrib
action of RIB peptide at were pre ound prot omassie B ound CRI
e input se ents.
he isolated Rac GTP 2001).
tion and d n vivo func
for the C of actin15 and the escence an ndom mov buted throu
the CRIB e was loa eloaded e teins were Blue. Mole B peptide et at 100%
d CRIB dom Pases and
dynamics o ctions of th CRIB dom 5 promote
localizatio nd live cell vement of ughout th
B domain ded onto ither with e separate ecular we e was qua
%. The data
main of co agrees w
of the coro he CRIB do main into t
r. This pla on of the
imaging. W Dictyosteli e cytosol
n with Ra columns
GDP or G ed on 15%
ight mark antified us
a represen
oronin has well with k
onin CRIB omain of co
he GFP e asmid (GF
coronin C We followe ium cells a and was
ac GTPas containin GTPS. Af
% polyacry kers are gi sing imag nt the ave
some pre known CR
B domain coronin, we
expression FP-cor-CRI CRIB doma
ed the loca and observ recruited
Results
19 ses. A.
g GST- fter one ylamide iven on geJ and rage of
eference RIB-Rac
e cloned n vector
IB) was ain was alization ved that to the
extend (Figure
Figure cor-CR micros selecte protrus wild ty images Additio restricte extend protrus the ent other h enriche (deHos 2.3 Inte Consid protein vitro. W Glutath inactive
ing pseudo e 12).
12: Loca RIB in AX scope for ed time p
sions is s ype coron s. Scale b
nally, we ed and tra ing pseud sion as sho tire cortica hand, the ed in the e stos et al., eraction o ering the s, we test We purified
hione-Seph e (RacGD
opod struc
lization of X2 cells. C
several m points are hown with nin in AX
ar, 5 μm.
have foun ansient. G dopod with own by the l region of full length entire pseu
1991;1993 of Dictyost presence ed the inte d GST fuse
harose be P) and ac
cture as sh
f the coro Cells were minutes in
e shown.
h arrowhe X2 cells. T
nd that the FP-cor-CR hin a few arrowhead f the pseud GFP-coro dopod alo 3; Gerisch
telium cor
e of a CR eraction of ed D. disco
ads and ctive (Rac
hown in the
onin CRIB e imaged n starvatio
. The rec ead. Right The lower
e transloca RIB was re seconds d in Figure dopod was onin, which ong with cy
et al.,1995 ronins wit RIB doma
f D. discoi oideum Ra
loaded th cGTP) form
e time-laps
domain.
with the on buffer.
cruitment t panels, lo r panels s
ation of G ecruited sp
after the e 12. This t s labeled w
h was rec ytosolic dis 5) (Figure 1 th Rac GT
in in the ideum Rac ac GTPase hem with
ms of Rho
se video m
Live cell confocal Represen of GFP-c ocalizatio show the
GFP-cor-CR pecifically
cell starte translocati with GFP-C
orded for stribution a
12).
Pases two Dicty c GTPases es express
GDP or G o GTPase
microscopy
imaging o l laser sc ntative ima
cor-CRIB on of GFP-
phase c
RIB was s to the rim ed to exte on continu Cor-CRIB.
compariso as reported
yostelium s with coro sed in E.co GTPS to s. These
20 images
of GFP- canning
ages at to the -tagged
ontrast
spatially m of the
end the ued until
On the on, was d earlier
coronin onins in oli using o mimic
in vitro
loaded washin to a m antibod In orde assay f family regulat and act Rac GT Rac1a and in nitroce Figure
GTPases g of beads membrane
dies.
er to test t for a know proteins a es severa tin polyme TPases, es
bound to cubated w llulose me
13, DGAP
s were the s, the prote which wa
the specifi wn interact and a kno
l cellular p rization. It specially o Glutathion with AX2 mbrane an P1 preferen
en incuba eins bound as probed
icity of the tion partne own effect processes
has been of the D. di ne-Sepharo
cell lysat nd probed ntially boun
ted with A d were sep
for bound
e assay, w er, DGAP1 ctor of D.
in D. disc shown to i iscoideum ose beads tes. The for DGAP1 nd to Rac1
AX2 cell l parated by d coronin
we first co . DGAP1 discoideu coideum li
interact pre Rac1 fam s was prelo
bound pro 1 with spec a loaded w
lysates an SDS-PAG proteins u
nducted a is a memb um Rac G
ke cell mo eferentially mily (Faix e
oaded with oteins wer cific antibo with GTPS
nd after re GE and tran using app
an in vitro ber of the GTPases.
otility, cyto y with GTP et al., 1998 h GDP or re transfe odies. As s
S.
Results
21 epeated nsferred ropriate
binding IQGAP DGAP1 okinesis, P loaded 8). GST-
GTPS erred to
hown in
Figure Sepha lysates on SDS using GST an
Figure bound incuba protein (de Ho GST an We ext Dictyos of GST
13: Rho rose bead s. After re S-PA gels
mAb 216 ntibodies.
14: Coro to Glutat ated with
ns were re ostos et al
ntibodies.
tended thi stelium sho T-Racs (Ra
GTPase ds was loa epeated w
, transferr -394-1. Th . The mole
nin-Rac in thione-Se
AX2 cell esolved by
l., 1991). G . Molecula
s pull-dow ort coronin acA, RacC
interactio aded with washing of red to nitr he GST fu ecular wei
nteraction pharose b lysates. A y SDS-PAG GST fusio ar weight m wn experim n with Rac , and RacE
on assay.
GDP or G f beads, t rocellulose
usion pro ights are i
n. GST, GS beads we After repe GE and pr ns were r markers in ment to inv GTPases E) bound t
GST-Rac GTPS an the bound
e membra oteins wer ndicated o
ST-RacA, re loaded ated wash robed for revealed b n kDa are vestigate t . Equal am to Glutathi
1a bound d incubat d proteins ane and pr re detecte on the left
GST-RacC with GDP hing of be coronin w by probing indicated the interac mounts of G
one-Sepha
d to Gluta ted with A s were sep
robed for ed by poly
t in kDa.
C and GST P or GTP eads, the with mAb g with poly
at the left ction speci
GST and a arose bead
22 athione- AX2 cell
parated DGAP1 yclonal
T-RacE S and bound 176-3-6 yclonal t.
ificity of a series ds were
incubat antibod GDP) (
Figure GST-R GTPS repeate nitroce protein membr The D.
propelle could in Rac pro
ted with AX dy. Coronin (Figure 14)
15: CRN Rac1a bou S and inc
ed wash ellulose m ns used i
rane. Mole discoideu er domain nteract wit oteins (A,
X2 cell lys n bound p ).
7-Rac inte und to Glu
cubated w ing of b membrane n the pul ecular wei um genome n (Shina e
h Rac GTP C, E and 1
ate. The b preferentia
eraction.
utathione- with AX2 beads, the and prob l down w ight marke e encodes
t al., 2010 Pases as w 1a) and, lik
bound prote lly to Rac
GST, GST -Sepharos
cell lysa e bound bed for C were detec
ers in kDa s a long co 0). We so well. GFP- ke coronin
eins were cC GTPase
T-RacA, G se beads w
ates expr proteins RN7 usin cted by P a are indic oronin isofo
ught out t -CRN7 sho
, it precipit
immunoblo e loaded w
GST-RacC were load ressing G s were tr g anti-GF onceau S cated at th orm (CRN7 to ascertai owed bindi tated prefe
otted with with GDP
C, GST-Ra ded with G GFP-CRN7
ransferred FP antibod S staining he left.
7) with tan in whether ng to all a erentially w
Results
23 coronin (RacC-
cE and GDP or 7. After
d to a dy. The
of the
ndem - r CRN7 nalysed with Rac
GTPas forms w In orde domain CRN7- GTPas repeat an incre A
B
ses loaded was retaine er to map th ns of CRN
CT). GFP- ses with va
preferred eased inte
with GDP ed (Figure
he binding 7 as indiv -CRN7-NT ariable affi the GTP l eraction wit
P. For Rac 15).
region of idual fragm T and GFP-
nities for t oaded form th RacC-G
cA and Ra
Rac in CR ments fuse -CRN7-CT the GDP o ms of Rac GTP and no
acE some
RN7, we ex ed to GFP T bound to or GTP loa cC and Rac o binding to
binding to
xpressed th (GFP-CR certain me aded forms
cE, GFP-C o RacC-GD
o the GTP
he two WD RN7-NT an
embers of t s. The C-t CRN7-NT
DP (Figure
24 loaded
D repeat d GFP- the Rac terminal showed e 16).
Results
25
Figure 16: CRN7-Rac interactions. A. GFP fusion deletion constructs of CRN7 is shown. The figure is not drawn to scale. Modified from Shina et al.,(2010). B.
CRNGST-Rac A, GST-Rac C, GST-Rac E and GST-Rac 1a bound to Glutathione- Sepharose beads were loaded with GDP or GTPS and incubated with AX2 cell lysates expressing either GFP-CRN7-NT or GFP-CRN7-CT. After repeated washing of the beads, the bound proteins were immunoblotted with anti-GFP antibody to detect GFP-tagged proteins. The GST fusions are shown in the lower panels. Molecular weight markers in kDa are indicated at the left.
From all the above experiments we conclude that D. discoideum coronin proteins (coronin and CRN7) preferentially interact with Rac GTPases in their GDP bound form. However, when the individual propellers of CRN7 are expressed, they prefer active Racs (GTP-loaded).
2.4.1 Coronin regulates myosin II function
Myosin II (conventional) in D. discoideum plays a central role in several cellular processes like cytokinesis, chemotaxis, and development. Myosin II can self- assemble into bipolar filaments. Phosphorylation of myosin II in its heavy chain regulates the dynamics of filament assembly (Bosgraaf and van Haastert., 2006) and impaired regulation of myosin II assembly and disassembly dynamics leads to severe cell polarity and developmental defects (Mondal et al., 2008).
We performed immunofluorescence analysis in order to elucidate the function of coronins in myosin II regulation. Myosin II typically localizes to the posterior cortex of chemotaxing cells where it helps to retract the cell body and suppress lateral pseudopod formation. It was observed that the amount of myosin II associated with the cytoskeleton increases and nearly doubles during the aggregation stage. When we stained coronin mutant cells for myosin II, we observed a similar distribution of myosin II in AX2 and corB mutant cells where we found an increased cortical staining only in aggregation competent cells. In contrast, corA- and corA-B- mutants showed an increased staining of myosin II in the cortex in vegetative cells comparable to the myosin II staining of aggregating cells (Figure 17).
Figure aggreg and st were t with im intensi
Figure ghosts
17: Dic gation com tained for
aken with mageJ us ity of myo
18: Coro s from veg
tyostelium mpetent A
myosin I h a confoc sing pseu osin II ove
nins regu getative a
m coronin AX2, corA-
I with mA cal micros do-3D pro r the scan
late myos and aggre
ns affect
-, corB-, a Ab56-396-5
scope. Th ojection p nned area.
sin assem egation co
myosin and corA-B
5 (Pagh a he confoca
plug-in. T .
mbly. Myos ompetent
assembly B- mutant nd Gerisc al stacks The z-axis
sin II level cells. The
y. Growin cells wer ch,1986).
were pro s represen
s in cytos e bar repr
26 ng and re fixed
Images cessed nts the
skeletal resents
Results
27
the mean of six independent experiments for vegetative and two for aggregating cells.
We further measured the amounts of myosin II recovered from detergent-insoluble cytoskeleton extracts of AX2 and coronin mutant cells. In the un-phosphorylated state, myosin II assembles into bipolar filaments and associates with the cytoskeleton. In AX2 cells, there is a twofold increase in the amount of myosin II recovered from cytoskeletal preparations of aggregation competent cells compared to growing cells reflecting the increased association of myosin II with the posterior cortical regions of chemotaxing cells. In contrast, corA- and corA-B- mutant cells showed an elevated level of myosin II in growing cells which was comparable to that in aggregating cells. On the other hand, cytoskeletal myosin II recovered from corB- was comparable to AX2 cells (Figure 18).
2.4.2 D. discoideum coronin interacts with Rac GTPases that regulate myosin II function
Rac GTPases regulate several cellular processes through CRIB containing effectors proteins. PAK kinases are such effector proteins that are regulated by Rac GTPases.
PAK kinases are activated by active Rac GTPases (GTP-bound), and in turn regulate downstream processes like myosin assembly (conventional myosin II) and myosin motor activity (unconventional myosin I) in the cell.
The D. discoideum genome encodes three canonical PAK kinases, PAKa, PAKb and PAKc. Two of these kinases, PAKa and PAKc, have been implicated in myosin II regulation (Chung and Firtel., 1999; Lee et al., 2004; Müller-Taubenberger et al.,2002). Additionally, these two PAK kinases are known targets for activated Rac GTPases, especially Rac1b and RacB. So we sought out to investigate whether coronin could interact with these two Rac GTPases. In pull-down assays we found that coronin bound preferentially to the GDP bound form of Rac1b and RacB (Figure 19).
Figure Rac1b and inc bound weight 2.5 Exp 2.5.1 C Effecto form of leads to (Lee et differen mutate the CR were m express interact
19: Coro bound to cubated w proteins t of coroni pression a Coronin CR or proteins f Rac GTP o the loss t al., 2004 nt coronin
d to alanin RIB (ISxP) w mutated (H
sed in AX tion in cellu
nin intera o Glutathio with cell ly were imm in is indic and chara RIB mutan
containin Pases. Mu
of Rac GT 4). To test proteins ne. In GFP
were muta 127A, V13 X2 and cor ular proces
acts with R one-Sepha ysates from munoblotte
cated on th cterizatio nt constru
g a CRIB utation or TPases bin
the impor in which P-MUT1 th ated and in 31A, G132A
rA mutant sses.
Rac1b and arose bead m AX2. Af ed with an he left.
n of coron ucts and e
domain b deletion o nding and rtance of t the conse he conserv GFP-MUT A) (Figure
cells to s
d RacB. G ds were lo fter repea nti-coronin
nin carryin xpression bind prefe of the CRI subseque this motif erved CRI ved residue
T2, the C-t 20A, B). T study the s
GST, GST- oaded with
ted washi n antibody
ng a muta n
rentially to B domain nt impaire in coronin B motif a es in the N terminal co These mut significanc
-RacB, an h GDP or ing of bea y. The mo
ated CRIB
o the GTP in such p ed protein f n, we crea amino acid
N terminal onserved re
tant protein ce of coro
28 d GST-
GTPS ads, the olecular
motif
P-bound proteins function ted two ds were part of esidues ns were nin-Rac
A
B
Figure the co stick m positiv shown residue mutate
20: A, Str oronin CR
model (re vely charg n. (B) Cor es in the ed to alani
ructure of RIB motif.
ed and b ged residu ronin CRI e N termi
ine.
f the D dis The muta blue). Only es adjace B mutant nal and C
scoideum ated resid
y side c ent to the C
t construc C termina
coronin C dues are
hains are CRIB dom cts used al part of
CRIB dom highlighte e shown main (R129 in our st f the CRI
ain. Side ed in a b for clarit 9, K130,) a tudy. Con IB domai
Results
29 view of all and ty. The are also nserved n were
In orde affects differen WT/AX Glutath The bo these prefere (Figure
Figure Rac1b and in GFP M were i The m GST (W
er to test if the Rac b nt mutant X2, GFP-M
hione-Seph ound prote assays, th entially to G e 21).
21: Inter bound to cubated w MUT2/ corA mmunobl membrane WB: GST).
f the muta binding ab proteins. T MUT1/corA harose col eins were he wild ty GDP-Rac1
raction of o Glutathio with cell l rA-cells. A otted with
was strip . The mole
tion of the ility, we ca To this end A- , and
umns con analyzed ype GFP
b while G
f CRIB m one Sepha lysates fr After repea
h anti-cor ped and r ecular wei
e conserve arried out a
d, equivale GFP-MU taining Ra by wester
coronin a FP MUT2
mutants w arose bead rom GFP-c ated wash ronin anti reprobed
ights are g
ed residues a binding ent amoun UT2/corA-
ac1b prelo rn blotting
and the G had comp
ith Rac G ds were lo cor WT/AX hing of be ibody mA
with poly given at th
s in the co assay with nts of lysat cells we aded with
with anti- GFP MUT pletely lost
GTPases.
oaded with X2, GFP M eads, the Ab 176-3-6
clonal an he left.
oronin CR h cells exp
tes from G ere loade
GDP or G GFP antib T2 protein
t its binding
GST, and h GDP or MUT1/corA
bound p 6 (WB: co ntibodies a
30 IB motif pressing GFP cor d onto GTPS.
body. In bound g ability
d GST- GTPS rA-, and proteins
oronin).
against