Diss. ETH No.:26921
Wetting, Bouncing, Drying and Freezing of Drops on Soft
Materials
A thesis submitted to attain the degree of Doctor ofSciences of ETH Zurich
(Dr. sc. ETH Zurich)
presented by
J ulia G erber
M. Sc. of Sciences , ETH Zurich born on: 20.11.1989 citizen of: Langnau i. E.
accepted on the recommendation of:
Prof. Dr. Dimos Poulikakos , examiner Prof. Dr. Doris Vollmer, co-examiner
Prof. Dr. Mark Tibitt, co-examiner Prof. Dr. Thomas Schuzius, co-examiner
2020
This document was typeset by the author using LATEXwith the typographical look-and-feelclassicthesis.
Julia Gerber :Wetting, Bouncing, Drying and Freezing of Drops on Soft Materials© June2020
A B S T R A C T
Interactions of liquids with soft materials occur abundantly in nature and technological applications, be it water wetting the surface of plant leaves, rain drops that impact on textiles, or liquids that are filtrated through membranes. Often these interactions involve complex simultaneous phase change phenomena, such as liquid-vapor and vapor-liquid transi- tions in drying, boiling, or condensation applications, or during freezing and melting. Traditionally, research focused on the interaction of liquids with rigid materials, largely overlooking the fact, that many relevant materials are soft, a fact that can significantly alter system behavior. Furthermore, employing thin layers of soft coatings to rigid materials can possibly result in desirable substrate properties, such as liquid repellency or low adhesion strength to ice. Hence, the knowledge base of wetting and interfacial phase change involving rigid materials, need to be significantly expanded into the area of soft materials, which is the focus of this thesis.
First, droplet drying on soft materials was investigated in depth and by focusing on microscopic level phenomena at the contact line region. We observed a transition towards lower contact angles, when the evaporative flux was high and the receding contact line speed exceeded a characteristic rate. We employed an advanced measurement technique, 4D reference-free traction force microscopy, to quantify the microscale substrate deformation field at the contact line, the so-called wetting ridge, and the related stress field during drying. We observed high and asymmetric local substrate v
speeds, leading to contact line motion retardation and in- ward tilting of the wetting ridge, resulting in smaller contact angles. These findings underpin a rate-dependent wettability on viscoelastic solids and are important for understanding liquid removal from soft materials and associated surface de- sign considerations. The developed methodology has great potential to study a wide range of complex dynamic soft substrate phenomena.
Next the focus of the dissertation shifted to the drying of suspension droplets on soft materials and the resulting particle deposit. One well-known type of particle footprint on rigid surfaces is the so-called “coffee ring”, that is when a particle ring is formed after the carrying liquid has evap- orated. In addition to lots of interesting physics, enhanc- ing or suppressing the “coffee ring” is relevant to printing, coating and microfabrication industries. We studied this phenomenon on soft materials and found that we can con- trol the topography of the deposit by simply adjusting the environment humidity, regulating the evaporative flux and receding contact line speed. By performing particle tracking, we discovered, that at low environment humidity advection of particles towards the contact line occurs, characteristic to the coffee ring effect, even for a non-pinned contact line, which we attribute to viscous dissipation in the soft sub- strate. Eventually, pinning occurred for expedited contact line speeds, resulting in a ring deposit as opposed to a cir- cular spot. We confirmed our findings in a contact printing configuration, showing the ability to trigger line bifurcation on soft substrates by regulating the evaporative flux.
The subsequent part of this dissertation focused on freez- ing of supercooled water and ice adhesion on soft materials.
This being a large research topic on its own, the results in this vi
study constitute a feasibility study. Soft coatings are known to have low adhesion strength to ice, which is why they are promisingicephobic materials and why understanding the mechanisms of ice detachment is important. We found, that when applying a shear force to an icicle attached to a soft material, very high substrate deformations and interfacial slippage occurred. On the other hand, when applying a normal, tensile force, we observed elastic instabilities, that are known to reduce the critical stress needed for fracture of the interface. Gaining knowledge of these distinct modes of ice removal from soft substrates will be the goal of future research activities.
Finally, we investigated how substrate flexibility can en- hance surface superhydrophobicity. We showed, that addi- tionally to the surface micro- and nanotexture, flexibility can boost liquid repellency. The underlying mechanism is the immediate acceleration and intrinsic responsiveness of flexi- ble materials to impacting droplets, mitigating the collision and lowering the impalement probability. These findings were confirmed on materials ranging from man-made (thin steel or polymer sheets) to natural (butterfly wings).
Taken together, the investigations presented in this thesis address important aspects of the broad and complex role of substrate compliance and flexibility in wetting and de- wetting of liquids, in particular in the presence of drying, and freezing. In doing so, the work makes a significant contribution to the knowledge base in this area and paves the way for future research activities and applications within the field.
vii
Wechselwirkungen zwischen Flüssigkeiten und weichen, fle- xiblen Materialien sind weit verbreitet in der Natur und in der Technik. So zum Beispiel benetzt der Morgentau die Oberfläche von Gräsern und Pflanzenblättern; in unserem Alltag versuchen wir uns mit wasserabweisender Kleidung vom Regen zu schützen und in der Wasseraufbereitung oder anderen technischen Prozessen spielt die Filtration von Flüssigkeiten durch Membrane eine wichtige Rolle. Oft ist bei diesen Prozessen auch ein Phasenwechsel involviert, sei es das Verdampfen, Kondensieren, Gefrieren oder Schmel- zen. Bisher wurde in der Forschung auf die Interaktion von Flüssigkeiten mit harten Oberflächen fokussiert, dabei wurde ausser Acht gelassen, dass viele Materialien weich oder flexibel sind. Diese Tatsache verändert das Zusammen- spiel von Flüssigkeit und Feststoff. Auch könnte die Auftra- gung von dünnen weichen Schichten auf harte Oberflächen zu gewünschten Oberflächeneigenschaften führen, wie bei- spielweise eine gute Wasserabweisung oder eine geringe Adhäsion zu Eis. Die Wissensgrundlage zu den Themen Benetzbarkeit und Phasenwechsel sollte daher erweitert wer- den, so dass flexible Materialien auch berücksichtigt werden, welches der Fokus dieser Dissertation ist.
Zunächst wurde die Verdampfung von Tropfen auf wei- chen Materialien untersucht. Wir beobachteten einen Über- gang zu niedrigeren Kontaktwinkeln, wenn die Verdamp- fungsrate hoch war und die Geschwindigkeit der Kontaktli- nie eine charakteristische Rate überschritt. Eine fortschrittli- che Messtechnik erlaubte es uns, die mikroskopischen Ver- viii
schiebungen im Substrate aufgrund der Oberflächenspan- nung und das damit verbundene Spannungsfeld während des Verdampfens zu quantifizieren. Wir stellten hohe und asymmetrische lokale Deformationen und Spannungen im Substrate fest. Diese führten zu einer Entschleunigung der Kontaktliniengeschwindigkeit und dem Einwärtskippen der Oberfläche bei der Kontaktlinie, was kleinere Kontaktwinkel zulässt. Diese Erkenntnisse zeigen auf, dass die Benetzbar- keit von viskoelastischen Materialien von der Geschwin- digkeit abhängt. Die entwickelte Methodik hat ein großes Potenzial zur Untersuchung vieler weiterer dynamischer Phänomene auf weichen Substraten.
Danach untersuchten wir das Verdampfen von Suspen- sionen auf weichen Oberflächen und die resultierenden Par- tikelablagerungen. Wir stellten dabei fest, dass bei hoher Luftfeuchte ein ausgefüllter, runder Abruck entstand, wo- hingegen sich bei niedriger Luftfeuchte, die Partikel an den Rändern des Abdrucks ablagerten, vergleichbar mit der Ab- lagerung die entsteht wenn Kaffee trocknet. Dieser Typ von Abdruck, wenn die Partikel sich an den Rändern sammeln, ist weit verbreitet und lange bekannt auf harten Oberflächen, und die Verstärkung oder Verhinderung dieses Effekts ist von Bedeutung für die Beschichtungs- und Mikrofabrika- tionsindustrie. Wir konnten nun feststellen, dass mithilfe von weichen Materialien die Advektion von Partikeln zu den Rändern der Flüssigkeit durch die Regulierung des Verdampfungsflusses kontrolliert werden kann. Bei tiefer Luftfeuchte wird die Bewegung der Kontaktlinie durch Dis- sipation im Substrat verlangsamt und gegen Ende des Ver- dampfungsprozesses vollends gestoppt, was zu einem aus- wärtsgerichteten Fluss führt, der resultierende Abdruck ist ein Ring.
ix
te sich auf das Einfrieren von unterkühltem Wasser und die Adhäsion von Eis auf weichen Materialien. Da es sich hierbei um ein umfassendes und eigenständiges Forschungs- thema handelt, ist dieser Teil der Dissertation lediglich als Machbarkeitsstudie zu betrachten. Aus neueren Studien ist bekannt, dass weiche Beschichtungen dazu führen, dass Eis mit ausserordentlich kleinen Kräften von Oberflächen entfernt werden kann, weshalb weiche Materialien als eis- abweisende Beschichtungen dienen könnten. Um solche Oberflächen implementieren zu können, ist das Ziel dieser Studie zu verstehen, welches die Mechanismen der Eisab- lösung sind. Wir beobachteten, dass die Ablösung von Eis unter Scherkräften zu sehr grossen Deformationen im Sub- strat und zum Gleiten zwischen den Grenzflächen führt.
Bei der Ablösung von Eis aufgrund von Zugkräften traten elastische Instabilitäten auf, von welchen bekann ist, dass sie die erforderliche kritische Spannung zur Initiierung eines Bruchs reduzieren. Die Untersuchung dieser unterschiedli- chen Arten der Eisablösung von weichen Oberflächen wird in zukünftigen Forschungsaktivitäten weiterverfolgt.
Schließlich untersuchten wir, wie die Flexiilität eines Sub- strats die Kapazität zur Wasserabweisung auf Oberflächen verändert. Wir konnten zeigten, dass zusätzlich zur Mikro- und Nanotextur der Oberfläche, die Flexibilität eines Sub- strats die Resistenz gegenüber aufprallenden Flüssigkeiten erhöhen kann. Der zugrundeliegende Mechanismus ist die sofortige Beschleunigung aufgrund auftreffender Tropfen und die intrinsische Reaktionsfähigkeit flexibler Materialien, wodurch der Aufprall gemildert und die Wahrscheinlichkeit eines Eindringens von Flüssigkeit in die Textur der Ober- fläche verringert wird. Wir bestätigten diese Erkenntniss sowohl an künstlich hergestellten (dünne Stahl- oder Poly- x
merplatten), wie auch an natürlichen Materialien (Schmet- terlingsflügel).
Zusammengefasst befassen sich die, in dieser Dissertati- on vorgestellten, Untersuchungen mit der komplexen Rolle der Flexibilität und Viskoelastizität des Substrates bei der Benetzung von Flüssigkeiten, insbesondere in der Gegen- wart von Verdampfungs- oder Gefrierungsprozessen. Damit leistet die Arbeit einen wesentlichen Beitrag zur Wissens- basis in diesem Feld und ebnet den Weg für zukünftige Forschungsaktivitäten und Anwendungen.
xi
A C K N O W L E D G M E N T S
First, I would like to thank Professor Dimos Poulikakos for giving me the possibility to conduct my doctoral studies in the laboratory of Thermodynamics in Emerging Technolo- gies. Besides sharing your long-term professional experience in research and encouraging me during my current work but also for future goals, you lightened up every meeting with your great sense of humor. This really made these four years a highly valuable experience for me.
I also thank Professor Thomas Schutzius, for the super- vision during my PhD. I learned a lot from you, and I was inspired by your creative way of solving problems or devel- oping new research questions. Your upright and cheerful manner motivated me time and time again and made this collaboration very pleasant.
Special thanks go to Professor Doris Vollmer and Professor Mark Tibbitt for taking their time and serving as my co- examiners.
Also I thank all my collaborators, especially Thomas Vasileiou and Tobias Lendenmann, for contributing to the successful publication of parts of this work.
I would like to thank the members of the LTNT group
—current and past ones. All of you created a very positive working environment and were eager to help if needed. This made my four years at LTNT an enjoyable time. I am also grateful for the technical support of Jovo Vidic and Peter Feusi.
I would like to acknowledge all of the Bachelor and Mas- ter students, that I worked with. Special thanks to Romy
xiii
who contributed to the successful completion of this work with their dedicated efforts.
Clearly this would not have been possible without the constant support of my family and friends, I am very grate- ful to all of you. Especially I thank my best friend, Martina Hodel, you were always there for me and your great ca- pability to analyze created new opportunities from every setback. I am grateful to my partner, Jan Wachtl, who made these four years a fantastic and adventurous time. And of course, I would like to thank my mother, Katharina Schären, who believed in me from the beginning and supported me during all my life. I wouldn’t be where I am without you.
Zurich , June2020
Julia Gerber
xiv
C O N T E N T S
1 i n t r o d u c t i o n 1
1.1 Context . . . 1
1.2 Thesis Outline . . . 3
2 f u n d a m e n ta l s 5 2.1 Surface Energy and Wettability . . . 6
2.1.1 Surface Energy . . . 6
2.1.2 Laplace Pressure . . . 8
2.1.3 Wetting States . . . 9
2.2 Viscoelasticity . . . 12
2.2.1 Stress - Strain Relation in the Linear Regime . . . 13
2.2.2 Maxwell Model . . . 16
2.3 Elastocapillarity . . . 17
2.3.1 Liquid Versus Elastic Interfaces . . . 18
2.3.2 Static Wetting on Soft Materials . . . . 19
2.3.3 Scales of Elastic Wetting and Effect on Apparent Contact Angle . . . 22
2.3.4 Dynamic Wetting on Soft Materials . . 26
2.4 Phase Change Phenomena . . . 29
2.4.1 Droplet Evaporation . . . 30
2.4.2 Droplet Freezing . . . 32
2.5 Colloid Science . . . 34
2.5.1 Colloid Stability and Interparticle Forces 35 2.5.2 Brownian motion . . . 40
2.5.3 Coffee Ring Effect . . . 41
2.6 Adhesion . . . 42
2.6.1 Physical Origin of Adhesion and Ad- hesion Energy . . . 43
xv
2.6.2 Theories of Adhesion of Two Elastic Surfaces . . . 43 2.6.3 Effect of Environmental Conditions on
Adhesion . . . 45 2.6.4 Adhesion on Soft Materials . . . 46 3 w e t t i n g t r a n s i t i o n s i n d r o p l e t d r y i n g
o n s o f t m at e r i a l s 49
3.1 Introduction . . . 51 3.2 Results . . . 53
3.2.1 Droplets drying on rigid and compli- ant substrates. . . 53 3.2.2 Transient deformation field detection
during droplet drying . . . 57 3.2.3 The effect of humidity and compliance
on droplet drying . . . 63 3.2.4 Wetting transitions in droplet drying
on compliant materials. . . 67 3.2.5 Mechanism of the dynamically trig-
gered wetting transition . . . 69 3.2.6 Displacement and tension in droplet
drying on soft materials. . . 71 3.3 Discussion . . . 75 3.4 Methods . . . 78
3.4.1 Sample preparation and material char- acterization . . . 78 3.4.2 Macroscopic evaporation experiments . 81 3.4.3 Traction force microscopy experiments
of droplets drying . . . 82 3.4.4 Reference-free cTFM algorithm . . . 83 3.4.5 Radial and vertical displacement and
effective line tension . . . 84 3.5 Supporting Information . . . 85 3.5.1 Reference-free cTFM measurement . . . 85
c o n t e n t s xvii
3.5.2 Considerations for Appropriate Mate-
rial Model Selection . . . 88
3.5.3 Force balance at contact line and strain dependence considerations . . . 88
3.6 Supporting Information Figures . . . 91
3.7 Supporting Information Tables . . . 99
3.8 Supporting Information Movies . . . .101
4 pat t e r n i n g o f c o l l o i d a l d r o p l e t d e p o s i t s o n s o f t m at e r i a l s 103 4.1 Introduction . . . .106
4.2 Results . . . .108
4.2.1 Effect of Environmental Conditions on Colloidal Patterns on Compliant ver- sus Rigid Materials . . . .108
4.2.2 Internal flows during droplet evapo- ration on a compliant material . . . . .112
4.2.3 Colloidal Transport and Assembly at the Liquid-Vapor Interface for lowrh condition on a Compliant Material . . .115
4.2.4 Discussion on Ring Formation dur- ing Colloidal Droplet Evaporation on a Compliant Material . . . .120
4.2.5 Bifurcation of Printed Lines on a Com- pliant Material . . . .121
4.3 Methods . . . .125
4.3.1 Sample fabrication and colloidal sus- pension preparation . . . .125
4.3.2 Colloidal Suspension Evaporation Ex- periments . . . .126
4.3.3 Analysis of Deposits . . . .127 4.3.4 Flow Field Measurements during Col-
loidal Suspension Droplet Evaporation 127
4.3.5 Detection of Particles in Three Dimen- sions . . . .128 4.3.6 Calculation of Binary Collision Events .129 4.3.7 Contact Printing with Colloidal Inks
Experiments . . . .133 4.4 Supporting Information . . . .134
4.4.1 Time Scale for Aggregation due to Floata- tion Forces . . . .134 4.4.2 Analysis of Deposit on a Compliant
Material at LowrhCondition . . . .135 4.4.3 The Importance of Marangoni Flows .137 4.5 Supporting Information Figures . . . .139 4.6 Supporting Information Movies . . . .145 5 d r o p l e t f r e e z i n g a n d i c e a d h e s i o n o n
s o f t m at e r i a l s 147
5.1 Introduction . . . .149 5.2 Results . . . .150
5.2.1 Microscale Substrate Deformation dur- ing Freezing . . . .150 5.2.2 Microscale Substrate Deformation as a
Result of Ice Shearing . . . .155 5.2.3 Microscale Substrate Deformation as a
Result of Normal Ice Detachment . . .160 5.3 Discussion and Outlook . . . .161 5.4 Methods . . . .163 5.4.1 Sample preparation . . . .163 5.4.2 Experimental Setup and Proceedure . .164 5.4.3 Material Characterization at Low Tem-
peratures . . . .165 5.4.4 Detection of Microscale Substrate De-
formation During Droplet Freezing . .166 5.4.5 Detection of Microscale Substrate De-
formation During Ice Removal . . . . .167
c o n t e n t s xix
5.5 Supporting Information Figures . . . .170
6 s u p e r h y d r o p h o b i c i t y e n h a n c e m e n t t h r o u g h s u b s t r at e f l e x i b i l i t y 173 6.1 Introduction . . . .176
6.2 Results . . . .179
6.2.1 Impalement Resistance . . . .179
6.2.2 Collision Outcome . . . .181
6.2.3 Butterfly wing . . . .183
6.3 Discussion . . . .185
6.4 Materials and Methods . . . .186
6.4.1 Droplet Impact Experimental Setup . .186
6.4.2 Laser Doppler Vibrometry . . . .186
6.4.3 Wettability Characterization . . . .186
6.4.4 Butterfly wings . . . .187
6.5 Supporting Information Figures . . . .188
6.6 Supporting Information Movies . . . .190
7 c o n c l u s i o n s 191 7.1 Results overview . . . .191
7.2 Outlook . . . .194 va r i a b l e s a n d a b b r e v i at i o n s 195
b i b l i o g r a p h y 203
l i s t o f p u b l i c at i o n s 221
c u r r i c u l u m v i ta e 223
1
I N T R O D U C T I O N
1.1 c o n t e x t
The interaction of liquids and solids is relevant to a wide range of biological and technical processes; from the percola- tion of water through soil, or the accumulation of drops on spider webs to the application of coatings to cars, airplanes and ships. Transpiration, that involves the evaporation from leaves, enables mass flow of mineral nutrients and water from roots to the upper parts of plants. The formation of clouds is initiated by condensation of small water drops on particles in the air. As seen from these two examples, many of the processes in which liquids are wetting solids, involve phase change. This intersection between wetting and phase change is a key factor in many energy related processes.
Condensation and evaporation are indispensable means of transferring heat in power generation or refrigeration cycles and prevention of ice formation on heat exchangers enables efficient operation.
This abundance of interesting and important processes, in- spired and motivated researches to contribute to the knowl- edge base within the field, already ranging back centuries.
One very influential study on wetting on rigid surfaces was performed by Thomas Young in the early 19th century [1].
Young found that there is an equilibrium angle that forms between a liquid deposited on a smooth, rigid solid, that is specific for a certain liquid and solid pair. By using his find-
1
ings, one can predict whether a liquid spreads on a smooth, rigid solid.
The development of microfabrication techniques created the possibility for surface engineering; this gave rise to many interesting research questions and enabled gaining control over certain liquid interactions with solids and interfacial phase change phenomena. Originally inspired by Lotus leaves [2], researches developed surfaces with hierarchical micro- and nanotexture, showing extreme liquid repellency and self-cleaning properties [3,4].
Traditionally, researchers focused on rigid materials, de- spite the fact that many biological or man-made materials are soft. Examples of such soft materials are plant leaves, textiles or polymers, which surround us in our daily life.
Therefore there is a need to study how liquids are wetting flexible, soft materials. A pioneering work was the study by George Lester, that showed theoretically how liquid drops can deform the underlying substrate, if the material has a low elastic modulus [5]. This fundamentally alters liquid wetting on soft substrates, compared to rigid ones. In this regard, the viscoelastic properties of the substrate have to be taken into account, which means that understanding the rheology of these materials is of importance. This field, com- bining elasticity and capillarity, has attracted a lot attention recently and is evolving rapidly. Researchers are starting to look into dynamic rather than static phenomena, show- ing that compliance can control droplet spreading [6–8], or enable collective droplet motion[9,10].
Furthermore, a few studies exist, looking at the effect of compliance on interfacial phase change phenomena. Re- search showed that compliance can enhance condensation [11], while other studies looked at ice repellency of soft ma- terials and found that flexibility enhances impact resistance
1.2 t h e s i s o u t l i n e 3
to supercooled droplets [12] and that soft materials have a very low adhesion strength to ice [13,14]. This makes soft coatings a promising candidate asicephobicmaterial, which could possibly bring an improvement of efficiency for many applications, such as wind turbines or ships, due to the prevention of ice accretion.
Further scientific advancements are needed, to under- stand how to employ soft materials, to develop or enhance superhydrophobicity or icephobicity, or improve droplet col- lection, and whether these properties prevail when the soft materials are applied as coatings onto rigid substrates. To make a significant contribution to this goal, combining the topics of mass transfer, heat transfer, thermodynamics with rheology and materials engineering is essential. This is the frame within my thesis is set out, aiming at gaining funda- mental understanding as well as influencing liquid-solid or solid-solid interaction.
1.2 t h e s i s o u t l i n e
This thesis first introduces the theoretical background, then four studies on wetting and phase change phenomena on soft materials are presented, followed by a discussion of the results. Specifically, the thesis is organized as follows.
c h a p t e r 2 - f u n d a m e n ta l s
This chapter presents the theoretical background necessary for the reader to understand the following chapters.
c h a p t e r 3-w e t t i n g t r a n s i t i o n s i n d r o p l e t d r y- i n g o n s o f t m at e r i a l s
In this chapter I present a study on droplet drying on soft materials, employing a microscopic view on the substrate deformation to explain the receding behavior of the contact line under varied environment humidity.
c h a p t e r 4- pat t e r n i n g o f c o l l o i d a l d r o p l e t d e- p o s i t s o n s o f t m at e r i a l s
In this chapter I present a study on suspension droplet dry- ing on soft materials, examining the effects of environment humidity and evaporative flux on the resulting footprint topography.
c h a p t e r 5- d r o p l e t f r e e z i n g a n d i c e a d h e s i o n o n s o f t m at e r i a l s
In this chapter I present a feasibility study on droplet freez- ing and ice adhesion on soft materials, measuring the micro- scopic deformation field of the substrate, also looking into the effects of the angle of attack of the force applied.
c h a p t e r 6 - s u p e r h y d r o p h o b i c i t y e n h a n c e m e n t t h r o u g h s u b s t r at e f l e x i b i l i t y
In this chapter I present a study on liquid repellency on flexible materials upon drop impact. This is a study, where my contribution focused on the superhydrophobicity of bio- logical samples (butterfly wings).
c h a p t e r 7 - c o n c l u s i o n s
Within this chapter I give an overview of the results and present a brief outlook.
2
F U N D A M E N TA L S
This chapter briefly introduces the fundamental background relevant to this thesis. This includes the topics of wetting and viscoelasticity and the field that combines both of these, elastocapillarity, as well as some of the basic concepts of phase change phenomena, colloid science and adhesion.
5
2.1 s u r f a c e e n e r g y a n d w e t ta b i l i t y
The field of wetting describes how liquids interact with solids. In this section, I describe the concepts of surface energy, Laplace pressure and wetting states. Parts of this section are based on the book "Capillarity and Wetting Phe- nomena" by de Gennes, Brochard-Wyart and Quéré [15].
2.1.1 Surface Energy
The liquid state is a condensed state in which molecules attract each other, with a force that is stronger than thermal agitation, but is weak enough that the molecules are still disordered. For lower attraction, molecules would change into the gas phase. A molecule in the bulk benefits from the interactions with all its neighbors (illustrated in Figure 2.1) and is situated in an energy minimum. By contrast, a molecule that is located at the surface of the liquid, is deprived of half of its cohesive interactions (illustrated in Figure2.1) because density is less in the vapor phase. Such a molecule is no longer in a favourable energy state. This is the fundamental reason, why liquids adjust their shape to obtain the smallest possible surface area. The surface energy, is a direct measure of this energy shortfall per unit surface area between molecules at the surface and molecules in the bulk.
Let us consider a typical scale for the surface energy of liquids. If the cohesion energy per molecule is Wcoh, then a molecule at the surface has an energy of roughlyWcoh/2.
Ifbis a molecule’s size andb2 is its exposed area, then the surface energy is on the order of γLV ∼= Wcoh/(2b2). For most oils, the interactions are of van der Waals type, which
2.1 s u r f a c e e n e r g y a n d w e t ta b i l i t y 7
liquid
vapor
solid θeq
γlv
γsv γsl
Figure2.1: A liquid droplet partially wetting a solid substrate.
Liquid molecules located in the bulk (light blue circle) experience interactions (red arrows) with all neighbor- ing molecules, whereas liquid molecules at the liquid vapor interface (dark blue circle) experience interac- tions to fewer neighboring molecules. This figure also illustrates the force balance of the interfacial tensions γLV,γSLandγSVat the contact line, Young’s law. The angle between the solid-liquid interface and the liquid vapor interface is the Young’s contact angle ,θeq. This illustration was inspired by Ref. [15].
means that Wcoh ∼ kBT; this results inγLV ∼=20 mJ m−2 at 25 °C. Water involves hydrogen bonds, it’s surface energy is therefore larger γLV ∼=72 mJ m−2. The subscript defines which phases are present at the considered interface, the subscript LV denotes the liquid-vapor interface. Likewise, the surface energy between two non-miscible liquids iand j is characterized by an interfacial energy γij.
An increase of a liquid surface area by an amount dA requires work. This work is proportional to the number of molecules that must be brought to the surface, that is todA.
The work required is:
δW = γdA. (2.1)
In this sense, γ is the energy that must be supplied to increase the surface area by one unit. Dimensionally,[γ] =
[EL−2]. The surface energy, is thus expressed in units of mJ m−2.
From a thermodynamic perspective,γis defined as the increase in free energy F that accompanies an increase in surface area:
γ= ∂F
∂A|T,V,n (2.2)
wherenis the number of molecules,T is the temperature andVis the total volume.
Surface energy can also be viewed as a force per unit length, then we usually talk aboutsurface tension. Dimension- ally,[γ] = [FL−1], and the surface tension is then expressed as mN m−1.
2.1.2 Laplace Pressure
As one passes across a curved surface or interface, a jump in pressure occurs. One can illustrate this using a spherical interface. Let us consider a case of a spherical drop of radius Rof a liquidiin another immiscible liquidj. If the interface between i and j is displaced by an amount dR, the work done by the pressure and capillary force can be written as
δW =−pidVi−pjdVj+γijdA, (2.3) with dVi = 4πR2dR = −dVj and dA = 8πRdR are the increase in volume and surface area, respectively, of the drop, pi and pj are the pressures in the liquidsiand j. The
2.1 s u r f a c e e n e r g y a n d w e t ta b i l i t y 9
condition for mechanical equilibrium isδW =0, which leads to
∆p= pi−pj = 2γij
R . (2.4)
The smaller a drop or bubble is, the larger the pressure there- fore is. The generalization of this argument is the Laplace theorem, which reads: The increase in hydrostatic pressure
∆pthat occurs upon traversing the boundary between two fluids is equal to the product of the surface tensionγ and the curvature of the surfaceK = R1
1 + R1
2,
∆p=γ 1
R1 + 1 R2
=γK, (2.5) whereR1 andR2are the radii of curvature of the surface.
2.1.3 Wetting States
Wetting is the study of how a liquid deposited on a solid or on another, immiscible liquid substrate spreads out. The phenomenon is pertinent to many technological applications, such as in chemical industry, automobile manufacturing and food processing.
2.1.3.1 The Wetting Parameter
A water droplet placed on a clean glass surface spreads completely. By contrast, the same water droplet placed on a sheet of plastic remains in place. The first example describes a completely wetting regime, whereas the second example describes a partial wetting regime. The parameter that sepa- rates those two regimes is called the wetting parameter,S,
which measures the difference between the surface energy per unit area of the substrate when dry and wet:
S = Es,dry−Es,wet
= γSV−(γSL+γLV) (2.6) For positive values ofS, the liquid spreads completely, in or- der to lower its surface energy. As a result a nanoscopic film establishes, whose thickness is determined by a competition between molecular and capillary forces.
For negative values ofS, the drop does not spread com- pletely, instead, at equilibrium it forms a spherical cap sitting on the substrate with a contact angle θeq.
2.1.3.2 Young-Dupré equation
Relating this equilibrium contact angle, θeq, to the interfacial energies was described in the pioneering work of Young and Dupré [1]. In this work, the equilibrium contact angle of a liquid drop on a smooth, rigid solid was derived by adding up the capillary forces at the contact line and equating the sum to zero, illustrated in Figure2.1. When normalized to a unit length, these forces are the interfacial energies/tensions between the solid, liquid and vapor phases. Projection of the equilibrium forces onto the solid plane, results in Young’s relation,
γLVcosθeq =γSV−γSL. (2.7) θeq can only be defined for negative values of S. The projection of the capillary forces onto the vertical axes is balanced by a reaction force exerted by the solid. If the solid is rigid, no substrate deformation is observable. If
2.1 s u r f a c e e n e r g y a n d w e t ta b i l i t y 11
the solid is soft, the substrate is deformed. This substrate deformation has far-reaching implications for the wetting on soft substrates, this will be described in detail in section 2.3.2.
2.1.3.3 Contact Angle Hysteresis
A liquid droplet on a clean, planar, rigid surface establishes a contact angle θeq, which is the equilibrium angle from Young’s law. Most often, the surfaces are not ideal, meaning that they contain defects, that are either chemical (such as stains) or physical inhomogeneities (such as surface rough- ness). On a non-ideal surface, the static contact angle is not unique. When inflating the droplet, the contact angle can exceed θeq without the contact line moving. Eventually, θ, reaches a threshold value,θa, beyond which the contact line starts to move. θa is defined as the advancing contact angle. Likewise, when deflating a drop, θ decreases again to a threshold value, θr, after which the contact line sud- denly shifts. θr is defined as the receding contact angle.
∆θ = θa−θr represents the contact angle hysteresis. The fundamental mechanism of hysteresis is, that during retrac- tion of a contact line over a surface defect, the contact line can pin locally on this defect forcing the contact line to stretch. Eventually, with enough force, the line breaks off, this is accompanied by a dissipation of energy.
2.1.3.4 Measurement Techniques for Contact Angles and Surface Energy
There are several methods for measuring the contact angle θeq. For relatively large angles, a side-view image can be taken to measure the angle directly. For angles less thanπ/4, an optical reflection technique is often used, alternatively an
interference method leads to higher precision. It is possible to calculate critical surface tension or surface free energy for a solid, γSV, by testing against a series of liquids and measuring the contact angles, by either probing a series of chemical compounds (n-alkanes, with n variable) or by using a polar and a non-polar liquid.
2.2 v i s c o e l a s t i c i t y
The science of rheology is that branch of mechanics which deals with the deformation and flow of matter. The theory of viscoelasticity is concerned with stress analysis involving materials that are neither purely elastic nor purely viscous.
In this section, I will explain what are the linear responses of purely elastic solids, viscous liquids and viscoelastic solids to a stress applied, also I will introduce the Maxwell model, that can be used to represent viscoelastic materials. I have based this section on the books "The Phenomenological The- ory of Linear Viscoelastic Behavior" by Nicholas W. Tschoegl [16], "Nonlinear Polymer Rheology" by Shi-Qing Wang [17], and "Understanding Viscoelasticity, An Introduction to Rhe- ology" by Nhan Phan-Thien and Nam Mai-Duy [18].
Upon applying a stress, a material being termed viscoelas- tic, stores part of the deformational energy elastically as potential energy, and simultaneously dissipates the rest through viscous forces. The rheological properties of vis- coelastic materials are time-dependent. In principle, all real materials are viscoelastic, the underlying idea is, that every- thing has a timescale and that if observation is done over a long enough period of time, then everything will flow.
From the rheological viewpoint, there is no clear distinction between solid and liquid; it is a matter between the rela-
2.2 v i s c o e l a s t i c i t y 13
tive timescale of the experiment, texp, to the timescaleτfor the material to fully develop a response. When those two timescale,texp andτ, are comparable, viscoelastic behavior can be observed. The condition is notably present in poly- meric materials which are thus the viscoelastic materials par excellence.
2.2.1 Stress - Strain Relation in the Linear Regime
Both ideal elastic solids and viscous liquids are known to exhibit linear response. Viscoelastic materials can also show linear response; however, only under sufficiently low magni- tude of strain and low rate of deformation.
e l a s t i c h o o k e a n s o l i d s The definition of an ideal, perfectly elastic solid is, that it undergoes a finite amount of deformation instantaneously when a stress,σ, is applied. For sufficiently small enough deformations, a linear relationship between the elastic deformation and the stress often exists.
σ(t) =Ge(t), (2.8) whereGis the shear elastic modulus ande(t)is the strain (in this case in shear).
v i s c o u s n e w t o n i a n l i q u i d Liquids differ from solids in that deformation can proceed indefinitely. In viscous liq- uids a deformation leads to instantaneous flow, whereas the flow of viscoelastic liquids is preceded by elastic defor- mation. Notably, flow has a very specific meaning, namely irrecoverable deformation. In contrast to flow, elastic defor- mation is recoverable, signifying that upon removing the
external stress, an elastically deformed sample returns to its non-deformed state. Let us consider the simplest case of simple shear in viscous liquids, then the stress is,
σ=ηe,˙ (2.9)
with the viscosity, η, and the strain rate, ˙e(in this case in shear). For viscous Newtonian liquids, ηis a constant and the shear stress is thus proportional to the shear strain rate
˙
e. Note, that equation2.9assumes a uniform velocity field with constant gradient, this is true for Newtonian liquids by definition. For polymeric liquids, this assumption is often not valid, except for very low shear rates.
v i s c o e l a s t i c r e s p o n s e s The generalization of the spe- cial cases of ideal elastic solids and ideal viscous liquids is treated in Boltzmann’s superposition principle for linear responses, describing viscoelastic behaviour. For viscoelastic materials, the concept of the elastic modulus, such as in equation2.8, is generalized by the time dependent modulus, G(t), which is typically a decreasing function in time. The case of a sudden small step strain, ∆e, made over a van- ishingly small period of time, ∆t, at timet1, illustrates the phenomenon of a viscoelastic response. The step in stress is then,
∆σ(t) =G(t−t1)∆e(t1), fort>t1. (2.10) According to Boltzmann’s superposition principle for linear response, a series of consecutive small step strains,
∆e(ti), should be additive in the resulting stressσ(t). If the
2.2 v i s c o e l a s t i c i t y 15
time steps are small enough, this leads to an integration, the stress is
σ(t) =
∑
n i=1G(t−t1) [(∆e/∆t)·∆t] =
Z −∞
t G(t−t0)e˙(t0)dt0, (2.11) witht1taken to be the time in the infinite past (−∞) and tn defines the present timet. This equation offers a general relationship between the strain history and stress, in the linear response regime, for all viscoelastic materials. For a startup shear at t=0 with a constant rate ˙e, equation2.11 can be rewritten as,
σ(t) =e˙ Z t
0
G(s)ds≡eη˙ (t). (2.12) For an oscillatory external deformation, one expects an oscillatory stress, in the linear response regime,
σ(t) ≡ σ0(ω)sin[ωt+δ(ω)]
= e0(G0sin(ωt) +G00cos(ωt))
≡ G0(ω)e(t) +G00(ω)e˙(t)/ω,
with the storage modulusG0 and the loss modulusG00. By definition tanδ ≡ G00/G0. According to this formula, the stress response, in the linear regime, is a combination of elastic deformation and viscous flow. The generic formula to relate the storage and loss modulus to the relaxation modulus are
G0 =ω Z ∞
0 G(s)sin(ωs)ds (2.13)
and
G00 =ω Z ∞
0 G(s)cos(ωs)ds. (2.14)
These formal relationships imply thatG0 andG00 contain the same information on the linear viscoelastic characteris- tics of the material system that the relaxation modulusG(t) does.
Stress relaxation after step strain is an elementary experi- ments to probe viscoelastic behavior. One imposes a sudden strain e, that occurs instantaneously at t = 0 and reaches the value ofe0att0. One then measures the residual stress σ(t)for t >t0. When the straineis sufficiently small, one observes linear response behavior, so that
σ(t) =G(t)e0. (2.15) The materials functionG(t)is known to be the relaxation modulus (as in equation2.11).
2.2.2 Maxwell Model
The Maxwell model treats viscoelastic materials, by assign- ing a spring with Hookean elastic constant Gand a dashpot with viscosityη, which are connected in series, as shown in Figure2.2(a). The elongation of the spring follows the law,
σ=Ges. (2.16)
The elongation of the dashpot is described by,
σ=ηe˙d. (2.17)
2.3 e l a s t o c a p i l l a r i t y 17
The total elongation of the material system is then the sum- mation of the two components, e= es+ed. By differentiat- ing equation2.16, one obtains
1 Gσ˙ + 1
ησ =e˙s+e˙d =e.˙ (2.18) If one performs a step-strain test on a material following a Maxwell model, one can use equation 2.18to obtain an expression for the stress σ(t). Namely, for a step-strain test, the strain rate, ˙e, is zero after reaching the strain of valuee0
att0. Solving this homogeneous differential equation leads to
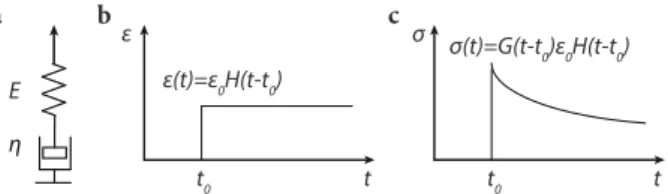
σ(t) =e0H(t−t0)G(t) =e0H(t−t0)G0exp(−t/τ), (2.19) with the heaviside function, H. Figure2.2(b) and (c) show the strain,e, vs.tand the stress,σ, vs.t, in such a step-strain test.
The Maxwell model offers the most useful and simplest phenomenological description for the stress relaxation in a viscoelastic material. In reality a viscoelastic material pos- sesses more than one elementary relaxation time. A gener- alized Maxwell model takes this into account and can be useful to describe linear viscoelastic processes in real sys- tems, consisting of multiple Maxwell elements (a spring and a dashpot in series), then arranged in parallel.
2.3 e l a s t o c a p i l l a r i t y
Elastocapillarity treats phenomena where capillary forces become relevant in comparison to elastic forces. This is
ε(t)=ε0H(t-t0) ε
t t0
σ(t)=G(t-t0)ε0H(t-t0) σ
t t0
η E
a b c
Figure2.2: Maxwell model for viscoelastic materials. (a) Schematic of a Maxwell element consisting of an elastic spring and a dashpot. (b) Strain,e, vs. time,t, in a step-strain test for a Maxwell material. (b) Stress,σ, vs. time,t, in a step-strain test for a Maxwell material.
the case for drops wetting fibers, very thin sheets or soft materials. In this section I will describe elastic interfaces as opposed to liquid interfaces and I will introduce concepts of static and dynamic wetting on soft materials. Parts of this section is based on the review papers by Style et al. [19], Chen et al. [20] and Adreotti and Snoeijer [21].
2.3.1 Liquid Versus Elastic Interfaces
As we have seen in Section2.1,thermodynamically interfaces are described by a surface energy, γij, which represents the excess free energy per unit area of an interface [15, 22].
Mechanically, this gives rise to a surface tension, Υij, which represents the excess force per unit length in the interface.
For liquids this tension is isotropic, such that one can write Υij = γijδij, with the Dirac delta functionδij. For an elastic interface, on the other hand, the surface tensionΥand the surface energyγare not equal. This phenomenon is termed the Shuttleworth effect [23] and evolves from the fact, that there are two distinct ways of creating new solid-vapor surface area. The first one is to separate two solid blocks
2.3 e l a s t o c a p i l l a r i t y 19
under constant strain, which is thus associated with the reversible energy, γ. The second way to create new solid- vapor interface area is by stretching the interface length by δL = L0δe. The associated work per unit length is given as ΥδL and involves surface tension Υ. The Shuttleworth relation then comes from equating this work to the increase of surface energy,δ(Lγ) =δ[L0(1+e)γ], which results in
Υ(e) = d
de[(1+e)γ(e)] =γ+ (1+e)dγ
de. (2.20) Concluding, the solid surface tensionΥinvolves a depen- dency on the change in surface energy during stretching, dγ/de.
Just as a fluid’s surface tension creates a jump in pres- sure across the curved interface (Young-Laplace equation), surface stress causes a jump in the stress across a solid interface. This stress anisotropy, σt, represents the excess tangential stress, or tension, localized in the interfacial zone.
It is therefore the difference between the stress component in the tangential direction, σtt, and in the normal direction, σzz,
σt =σtt−σzz. (2.21) The integral of σt across the interface gives the interfacial force per unit length, which is the solid surface tension,Υ.
2.3.2 Static Wetting on Soft Materials
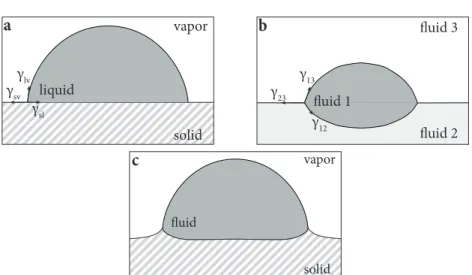
As we have seen in Section2.1, a droplet deposited on a rigid substrate fulfills Young’s law, signifying that the angle made at the contact line reflects a balance of the surface energies
(see Figure2.3(a)). In contrast, a droplet floating on another, immiscible liquid, does strongly deform the interface with contact angle’s determined by Neumann’s law (see Figure 2.3(b)). Specifically, the angles between all three interfaces are determined by a vectorial balance of the surface tensions at the contact line,
Υ12·~t12+Υ13·~t13+Υ32·~t23=0, (2.22) where the surface stress tensor and the tangent vector of the interface between phasesiandjare denoted byΥij and
~tij.
Soft compliant materials represent the intermediate situ- ation, in between those two extreme cases. A droplet on a compliant substrate does deform the underlying substrate due to capillarity; however in contrast to a liquid the compli- ant substrate does exhibit an elastic resistance. The Laplace pressure inside the droplet, 2γ/R, causes a depression of the surface profile beneath the droplet and the out-of-plane component of the surface tension,Υsinθ, pulls the substrate up at the contact line and creates a localized deformation of the substrate, the so-called wetting ridge (see Figure 2.3(c)).
Let us consider a straight contact line on a semi-infinite elastic substrate. The force per unit length, hereγLVsinθ, is spread over a width of the order of the molecular size, b.
Given that there are significant deformations at the contact line, let us consider the force balance between bulk and surface stresses on a small test volume around the contact line (see Figure2.4),
Z
W1
σ1·~n1dL+
Z
W2
σ2·~n2dL+
Z
W3
σ3·~n3dL +Υ12·~t12+Υ13·~t13+Υ32·~t23 =0.
(2.23)
2.3 e l a s t o c a p i l l a r i t y 21
liquid
vapor
solid γsvγlv
γsl
a
solid fluid
vapor
c
fluid 1
fluid 2 fluid 3
γ23 γ13 γ12
b
Figure2.3: Wetting on rigid substrates, liquids and soft substrates.
(a) A droplet sitting on a rigid substrate fulfills the Young-Dupré law, in this caselec b. (b) A droplet sitting on an immiscible fluid fulfills Neumann’s tri- angle, in this caselec bandlec R. (c) A droplet sitting on a soft substrate, representing the intermedi- ate case, in this caselec bandlec R. Reprinted (adapted) with permission from Ref. [19]. Copyright 2015, Springer Nature.
σ3.n3
σ1.n1 σ2.n2
Υ13 .t13 Υ23 .t23
Υ12 .t12
Figure2.4: Force balance at the contact line of a droplet on a soft solid on a small volume (with sizeb). Figure reproduced from [19].
When shrinking the test volume, the contribution of the bulk stresses vanishes, whereas those from the surface stress remain finite, thus recovering Neumann’s vector balance (equation 2.22). This signifies that at close distances from the contact line, capillarity dominates and that the contact angles at the apex of the wetting ridge fulfill Neumann’s law.
Most work focussing on the theory of static wetting on soft materials used linear elastic theory to calculate the substrate deformation profile caused through a sessile droplet [24–27].
The requirement for linear elastic theory to be valid, is that strains are small.
2.3.3 Scales of Elastic Wetting and Effect on Apparent Contact Angle
In order to reliably describe elastocapillary phenomena, a classification in terms of length scales is needed. For a solid surface with mean curvature, K, and isotropic, uniform surface tension, Υ, the jump in normal stress across a solid
2.3 e l a s t o c a p i l l a r i t y 23
interface is simply KΥ. For an elastic solid with Young’s modulus,E, this stress jump leads to local deformation in the bulk solid. Stresses balance so that KΥ∼ Ee. One expects significant deformations (e ∼ 1) when 1/K ∼ Υ/E. This defines the elastocapillary length,Υ/E, which separates the small scales dominated by capillarity from the large scales dominated by elasticity. In the case of a liquid on a soft solid Υ=γLVand the elastocapillary length is thus given as,lec,
lec =γLV/E. (2.24)
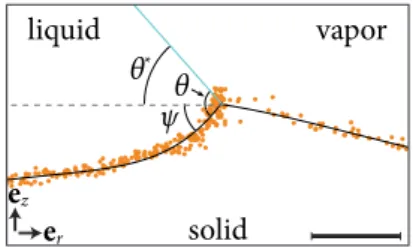
The question arises, when the deformation at the contact line becomes significant, so that the apparent contact angle is deviating from the Young’s contact angle. Note, that there is a distinction between the apparent contact angle,θ∗lv, that is detectable macroscopically and the contact angle,θLV. Figure 2.5(a) presents a close-up view of the contact line of a droplet sitting on a rigid surface, for this case the definition of the contact angle is unmistakable. For a droplet sitting on a soft material or another immiscible liquid we define the appar- ent contact angle, θlv∗, as the angle between the liquid-vapor interface and the horizontal (of the undeformed substrate), see close-up views of the contact lines of a droplet sitting on a soft material in Figure 2.5(b) and a droplet sitting on another immiscible liquid 2.5(c). Now, to define these three wetting regimes (rigid, soft or liquid), one must compare the elastocapillary length,lec, to other length scales of the system, such as the nanometric interface width, that is the molecular sizeb, and to macroscopic parameters such as the drop size Rand the thicknessh of the substrate. The rigid limit is when γLV/E b, then the excess stress γLV/b is negligible compared to Eand cannot induce any significant deformation below the contact line (see Figure2.5(a)). In the
θs
θlv
θs θs
a ξ
θ*lv θ*lv
b c
Figure2.5: Transition of contact angles from rigid to soft ma- terials. Schematics of the angles at the contact line for, (a),γLV/(ER) 1, (b),γLV/(ER) ≈ 1 and, (c), γLV/(ER)1. Reprinted (adapted) with permission from Ref. [28]. Copyright (2014) Cambridge University Press.
intermediate case,b γLV/E R, surface tension domi- nates at small scales and a sharp wetting ridge forms; on the scale of the drop, however, elasticity still dominates and the liquid angle remains unperturbed; Young-Dupré holds (see Figure 2.5(b)). On the other hand, when γlv/E R, capillarity dominates overall and the drop takes the shape of a liquid lens (see Figure2.5(c)). There is a smooth transition from the apparent contact angle from the Young-Dupré to the Neumann limits with droplet size, asγLV/(ER)is grow- ing larger than1. This deviation from Young-Dupré angle, ξ > 0, is caused through a rotation of the cusp. However, the shape near the contact line is universal, because the surface-stress dominated regime near the contact line has a width ofγLV/E. This signifies, that the solid angle,θs, is constant and only depends on the solid surface tension,Υs. Theoretical derivations for the calculation of the appar- ent contact angle of liquids on soft materials exist. Most often these studies perform a minimization of the elastic and capillary energies to find the profiles of the solid-vapor,
2.3 e l a s t o c a p i l l a r i t y 25
Figure2.6: X-ray image of a wetting ridge of a droplet on a silicone gel (E=3 kPa). Scale bar, 5 µm. Reprinted (adapted) with permission from Ref. [30]. Copyright 2014, Springer Nature.
.
liquid-vapor and solid liquid interfaces and with this the angles with which these interfaces meet at the contact line [6,7,24–26,28,29]. These studies make simplifying assump- tions, such as linear elastic behavior and symmetric surface energies (Υsv = Υsl = Υs). The findings in these papers present some conflicting information, thus the issue is not resolved conclusively.
In the intermediate case of Figure2.5(b), whenγLV/(ER)≈ 1, the wetting ridge is shown to be symmetric. However, in equilibrium this is only the case for symmetric solid surface tensions,Υsl=Υsv. If the solid surface tensions are not sym- metric, one observes a rotation of the wetting ridge, such as shown in Figure2.6.
2.3.4 Dynamic Wetting on Soft Materials 2.3.4.1 Spreading of Droplets on Soft Materials
When a liquid droplet is deposited on a rigid material the un- balanced capillary force, γLV(cos(θ)−cos(θeq)), drives the liquid to spread on the surface until equilibrium is reached.
This spreading mechanism passes through three phases, withR(t)following a power law in time in each phase. The first two phases are essentially inertia dominated, whereas the third phase is dominated by liquid viscosity, the spread- ing speed is decreasing in time. When a liquid droplet is deposited on a soft substrate, it is forming a wetting ridge, which has to move together with the contact line and this leads to additional dissipation, due to the viscoelastic nature of the substrate. In the following, I will discuss how the formation and motion of the wetting ridge influences the spreading velocity as well as the advancing contact angle.
Experimental studies have found two phases of spreading, leading to distinct regimes for the spreading velocity for a droplet on a soft material. In the first few milliseconds after droplet deposition, the spreading is fast, following the same power law as on rigid substrates [8]. Then follows a second, slower phase, where R approaches a maximum value, which is distinct for different degrees of compliance.
Figure 2.7(a) shows the spreading of liquid droplets on sub- strates with varying shear modulus [8], showing the two dis- tinct spreading regimes and the slowing down of spreading due to substrate viscoelasticity. In the second, viscoelasticity dominated spreading regime, the contact line velocity was found to be in the range of v ∼ 10−4−10−3 m s−1 [6–8, 32]. Based on an energy balance between capillary driving force and the viscous dissipation in the solid, the relation
2.3 e l a s t o c a p i l l a r i t y 27
r (mm)
v
1 1/2
10–6 10–5 10–4 10–3 10–2 10–1 1 10–1
1 101 102
Time
a b
t (ms) (mm s-1)
ϕ (°) ϕmax
ϕcrit
Figure2.7: Spreading of droplets on viscoelastic substrates. (a) Spreading radii of water drops (color symbols) on different viscoelastic substrates and asymptotic fits to the inertial regime (solid lines,Rfollows a power law int) and viscoelastic regime (dashed lines,Rap- proaches exponentially a maximum value). The shear moduli,G, of the substrates were:G= 679 kPa (),G
= 204 kPa (◦),G= 5.8 kPa (4),G= 1.8 kPa (5) andG
= 0.02 kPa (♦). Reprinted (adapted) with permission from Ref. [8]. Copyright (2013) American Chemical Society. (b) Dynamic angleφ= θ∗−θeq∗ for water on the silicone gel (symbols). Data are averaged over10 independent experiments, error bars represent the s.d.
The small-v behaviour exhibits the same power-law as G00. Solid line corresponds to theoretical calcula- tion ofφ, describing the full range of velocities. The critical angle of depinningφcrit=39°±3°, measured separately, is plotted at an arbitrary velocity. Reprinted (adapted) with permission from Ref. [31]. Copyright 2015, Springer Nature.
(cos(θ)−cos(θeq)) ∼ (v/v∗)m, with a characteristic speed related to the rate dependent viscoelastic dissipation of the soft material,v∗. The exponentmis linked to the viscoelastic properties of the substrate and was found to be of the order of 0.5∼ 0.6 in experiments, for droplets spreading on soft materials [6,7,32].
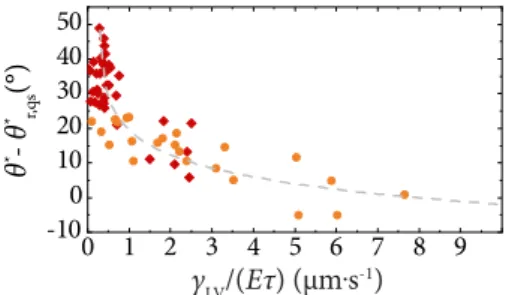
In Ref. [31], the authors studied the apparent contact angle of liquid droplets spreading on viscoelastic substrates as a function of contact line velocity. They reasoned that the increase in apparent contact angle, θ∗, is a result of a rotation of the wetting ridge by angleφ, withθ∗ =θ∗eq+φ.
Figure 2.7(b) showsφvs. the contact line velocity, v, for a water droplet on a silicone substrate. From this, we see that the wetting ridge rotation angleφis increasing withv, but levels off at higher velocities, approaching a critical value.
The dependency of the wetting ridge rotation on the vis- coelastic substrate properties was found, φ∝(v/v∗)m, with the characteristic velocity scalev∗= γS/(Gτ)[31,33]. This is basically a similar relation that was found by Refs. [6,7, 32], but this time obtaining an expression forv∗.
2.3.4.2 Stick-Slip Motion of Moving Contact Lines on Soft Ma- terials
A liquid spreading on a viscoelastic material can also show distinct modes of advancing, a continuous motion and a so- called stick-slip motion [34]. In Ref. [34], the authors tested these spreading regimes on millimetric thick samples of gels, cross-linked network of polymers swollen with solvent and they found, that at low and very high inflation rates, the contact line motion is continuous, whereas at intermediate inflation rates, the contact line performs a stick-slip motion.
This signifies that the contact line pins regularly during