ζ . Kristallogr. N C S 2 1 3 ( 1 9 9 8 ) 7 8 7 - 7 8 8 7 8 7
© by R. O l d e n b o u r g V e r l a g , M ü n c h e n
Crystal structure of l,7-dihydroxytricyclo[5.3.1.1^'^]dodeca-4,9-dien- 3,8,ll,12-tetraone, C20H24O6
W. Krämer, E. Geyer and D. Schollmeyer
Universität Mainz. Institut für Organische Chemie, Saarstraße 21. D-55099 Mainz, Germany
Received June 2, 1998, CSD-No. 409315
Fig. 1. One of the three independent molecules.
Fig. 2. Projection of the unit cell, view along [100].
S o u r c e of m a t e r i a l : T h e o x i d a t i o n o f 4 , 6 - d i e t h y l p y r o g a l i o l l e a d s - in d e p e n d e n c e o n t h e c o n d i t i o n of t h e r e a c t i o n - t o d i f f e r e n t d i m e r 3 - h y d r o x y - o - q u i n o n e s . In all it e x i s t s 4 d i f f e r e n t s t r u c t u r e s ( s e e r e f . 1 ). B y u s i n g p o t a s s i u m n i t r o s o d i s u l f o n a t e in a q u e o u s m e d i a a t p H = 8 l , 7 - d i h y d r o x y t r i c y c l o [ 5 . 3 . 1 . 1 ^ ' ^ d o d e c a - 4 , 9 4 l i e n - 3 , 8 , 1 1 , 1 2 - t e t r a o n e will b e o b t a i n e d . C r y s t a l s w e r e o b t a i n e d t h r o u g h s l o w c r y s t a l l i z a t i o n f r o m e t h e r . T h e p o s i t i o n of t h e c a r b o n y l b r i d g e s h a v e b e e n c l a r i f i e d b y X - r a y d i f f r a c t i o n .
С2(Л240б, triclinic, PI ( N o . 2), α =9.1321(1) Â, ¿? =12.4632(2) Λ , с = 1 3 . 9 1 3 4 ( 3 ) Â , α =109.363(2)°, β =1(Ϊ7.123(2)°, γ= 9 4 . 7 4 2 ( 2 ) ° , ν = 1 3 9 9 . 0 Â ^ Ζ = 3 , R(F} =0.(Μ5, Ry^F^) = 0 . 1 3 2 .
Table 1. Parameters used for the X-ray data collection
Ciystal: colorless block, size 0.26 χ 0.26 χ 0.42 mm Wavelength: Cu «Γα radiation (1.54180 Â)
μ: 7.80 cm"'
Diffiractometer: Enraf-Nonius CAD4 Scan mode: οι/2β Tmeasurrnuiu'· 298 К
2θ™,χ: 140.04°
5286
Criterion for/о: / ο > 2 σ ( / ο ) Ν(ραΓαηι)Γφκά: 379
Programs: SIR92, SHELXL-97, CELSIUS, Corine
Table 2. Final atomic coordinates and displacement parameters (in A^)
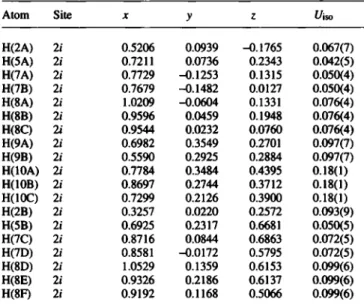
Atom Site X У ζ í/iso
H(2A) 2/ 0.5206 0.0939 -0.1765 0.067(7) H(5A) 2i 0.7211 0.0736 0.2343 0.042(5) H(7A) 2i 0.7729 -0.1253 0.1315 0.050(4) H(7B) 2i 0.7679 -0.1482 0.0127 0.050(4) H(8A) 2i 1.0209 -0.0604 0.1331 0.076(4) H(8B) 2i 0.9596 0.0459 0.1948 0.076(4) H(8C) 2i 0.9544 0.0232 0.0760 0.076(4) H(9A) 2i 0.6982 0.3549 0.2701 0.097(7) H(9B) 2i 0.5590 0.2925 0.2884 0.097(7) H(IOA) 2i 0.7784 0.3484 0.4395 0.18(1) H(IOB) 2i 0.8697 0.2744 0.3712 0.18(1) H(IOC) 2i 0.7299 0.2126 0.3900 0.18(1) H(2B) 2i 0.3257 0.0220 0.2572 0.093(9) H(5B) 2i 0.6925 0.2317 0.6681 0.050(5) H(7C) 2i 0.8716 0.0844 0.6863 0.072(5) H(7D) 2i 0.8581 -0.0172 0.5795 0.072(5) H(8D) 2i 1.0529 0.1359 0.6153 0.099(6) H(8E) 2i 0.9326 0.2186 0.6137 0.099(6) H(8F) 2i 0.9192 0.1168 0.5066 0.099(6)
788
C20H24O6Table 2. (Continued) Table 2. (Continued)
Atom Site X У ζ t/iso Atom Site X у ζ i/iso
H(91A) 2i 0.5533 0.3783 0.6604 0.102(9) Н(5С) 2/ 0.3111 0.4363 0.0910 0.040(5)
H(91B) 2i 0,4971 0.3799 0.5433 0.102(9) Н(7Е) li 0.2123 0.5162 -0.1409 0.048(4)
H(92A) 2i 0.3689 0.3275 0.5773 0.102(9) H(7F) 2i 0.3533 0.5435 -0.0340 0.048(4)
H(92B) 2i 0.5351 0.3688 0.6651 0.102(9) H(8G) li 0.3761 0.3853 -0.1720 0.073(4)
H(IOD) 2i 0.3105 0.4180 0.6190 0.171 Н(8Н) li 0.2130 0.3175 -0.1876 0.073(4)
H(IOE) 2i 0.3049 0.2997 0.6360 0.171 Н(81) li 0.3546 0.3451 -0.0806 0.073(4)
H(IOF) 2i 0.2484 0.3006 0.5185 0.Ι7Ι Н(9Е) li 0.2912 0.3330 0.2009 0.064(5)
H(llD) 2i 0.4810 0.4868 0.5891 0.160 H(9F) li 0.1413 0.2346 0.1509 0.064(5)
H(llE) 2i 0.4533 0.3909 0.4753 0.160 H(IOG) li 0.1741 0.3434 0.3308 0.125(7)
H(llF) 2i 0.6207 0.4325 0.5637 0.160 H(IOH) li 0.0183 0.3580 0.2550 0.125(7)
H(2C) 2i -0.3212 0.2791 -0.0968 0.084(8) H(IOI) li 0.1680 0.4565 0.3050 0.125(7)
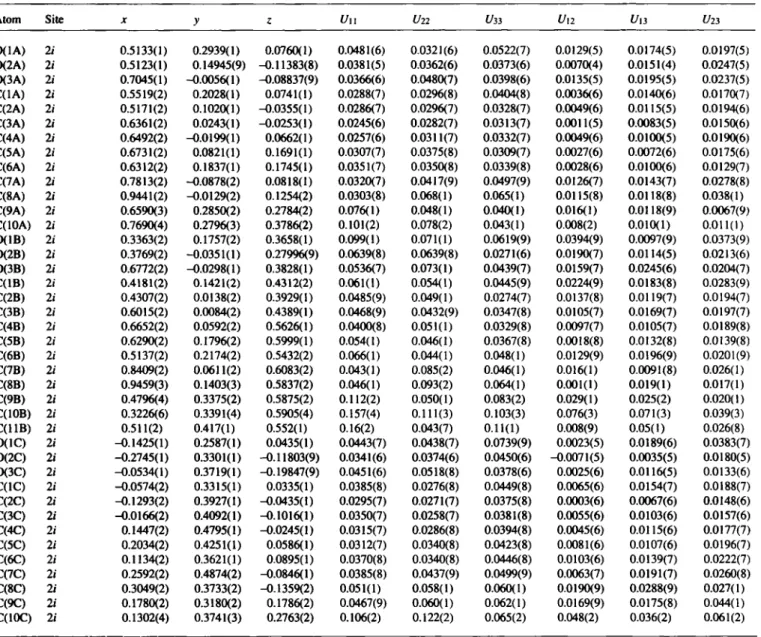
Table 3. Final atomic coordinates and displacement parameters (in Â^)
Atom Site X У ζ Un f/22 Í/33 Ul2 U,3 U23
2У 2i 2i li 2i 2i 2i 2i CKIA) 0(2A) 0(3A) C(1A) C(2A) C(3A) C(4A) C(5A)
C(6A) 2i
C(7A) 2i C(8A) C(9A) C(IOA) 2/
0(1B) 2i
0(2B) 0(3B) C(1B) C(2B) C(3B) C(4B) C(5B) C(6B) C(7B) C(8B) C(9B) C(IOB) 2i C(llB) 2i 0(1C) 2Í 0(2C) 0(3C) C(1C) C(2C) C(3C) C(4C) C(5C) C(6C) C(7C) C(8C) C(9C)
2/
2i
C(IOC) 2i
0.5133(1) 0.5123(1) 0.7045(1) 0.5519(2) 0.5171(2) 0.6361(2) 0.6492(2) 0.6731(2) 0.6312(2) 0.7813(2) 0.9441(2) 0.6590(3) 0.7690(4) 0.3363(2) 0.3769(2) 0.6772(2) 0.4181(2) 0.4307(2) 0.6015(2) 0.6652(2) 0.6290(2) 0.5137(2) 0.8409(2) 0.9459(3) 0.4796(4) 0.3226(6) 0.511(2) -0.1425(1) -0.2745(1) -0.0534(1) -0.0574(2) -0.1293(2) -0.0166(2) 0.1447(2) 0.2034(2) 0.1134(2) 0.2592(2) 0.3049(2) 0.1780(2) 0.1302(4)
0.2939(1) 0.14945(9) -0.0056(1)
0.2028(1) 0.1020(1) 0.0243(1) -0.0199(1) 0.0821(1) 0.1837(1) -0.0878(2) -0.0129(2) 0.2850(2) 0.2796(3) 0.1757(2) -0.0351(1) -0.0298(1) 0.1421(2) 0.0138(2) 0.0084(2) 0.0592(2) 0.1796(2) 0.2174(2) 0.0611(2) 0.1403(3) 0.3375(2) 0.3391(4) 0.417(1) 0.2587(1) 0.3301(1) 0.3719(1) 0.3315(1) 0.3927(1) 0.4092(1) 0.4795(1) 0.4251(1) 0.3621(1) 0.4874(2) 0.3733(2) 0.3180(2) 0.3741(3)
0.0760(1) -0.11383(8) -0.08837(9) 0.0741(1) -0.0355(1) -0.0253(1) 0.0662(1) 0.1691(1) 0.1745(1) 0.0818(1) 0.1254(2) 0.2784(2) 0.3786(2) 0.3658(1) 0.27996(9) 0.3828(1) 0.4312(2) 0.3929(1) 0.4389(1) 0.5626(1) 0.5999(1) 0.5432(2) 0.6083(2) 0.5837(2) 0.5875(2) 0.5905(4) 0.552(1) 0.0435(1) -0.11803(9) -0.19847(9) 0.0335(1) -0.0435(1) -0.1016(1) -0.0245(1) 0.0586(1) 0.0895(1) -0.0846(1) -0.1359(2) 0.1786(2) 0.2763(2)
0.0481(6) 0.0381(5) 0.0366(6) 0.0288(7) 0.0286(7) 0.0245(6) 0.0257(6) 0.0307(7) 0.0351(7) 0.0320(7) 0.0303(8) 0.076(1) 0.101(2) 0.099(1) 0.0639(8) 0.0536(7) 0.061(1) 0.0485(9) 0.0468(9) 0.0400(8) 0.054(1) 0.066(1) 0.043(1) 0.046(1) 0.112(2) 0.157(4) 0.16(2) 0.0443(7) 0.0341(6) 0.0451(6) 0.0385(8) 0.0295(7) 0.0350(7) 0.0315(7) 0.0312(7) 0.0370(8) 0.0385(8) 0.051(1) 0.0467(9) 0.106(2)
0.0321(6) 0.0362(6) 0.0480(7) 0.0296(8) 0.0296(7) 0.0282(7) 0.0311(7) 0.0375(8) 0.0350(8) 0.0417(9) 0.068(1) 0.048(1) 0.078(2) 0.071(1) 0.0639(8) 0.073(1) 0.054(1) 0.049(1) 0.0432(9) 0.051(1) 0.046(1) 0.044(1) 0.085(2) 0.093(2) 0.050(1) 0.111(3) 0.043(7) 0.0438(7) 0.0374(6) 0.0518(8) 0.0276(8) 0.0271(7) 0.0258(7) 0.0286(8) 0.0340(8) 0.0340(8) 0.0437(9) 0.058(1) 0.060(1) 0.122(2)
0.0522(7) 0.0373(6) 0.0398(6) 0.0404(8) 0.0328(7) 0.0313(7) 0.0332(7) 0.0309(7) 0.0339(8) 0.0497(9) 0.065(1) 0.040(1) 0.043(1) 0.0619(9) 0.0271(6) 0.0439(7) 0.0445(9) 0.0274(7) 0.0347(8) 0.0329(8) 0.0367(8) 0.048(1) 0.046(1) 0.064(1) 0.083(2) 0.103(3) 0.11(1) 0.0739(9) 0.0450(6) 0.0378(6) 0.0449(8) 0.0375(8) 0.0381(8) 0.0394(8) 0.0423(8) 0.0446(8) 0.0499(9) 0.060(1) 0.062(1) 0.065(2)
0.0129(5) 0.0070(4) 0.0135(5) 0.0036(6) 0.0049(6) 0.0011(5) 0.0049(6) 0.0027(6) 0.0028(6) 0.0126(7) 0.0115(8) 0.016(1) 0.008(2) 0.0394(9) 0.0190(7) 0.0159(7) 0.0224(9) 0.0137(8) 0.0105(7) 0.0097(7) 0.0018(8) 0.0129(9) 0.016(1) 0.001(1) 0.029(1) 0.076(3) 0.008(9) 0.0023(5) -0.0071(5) 0.0025(6) 0.0065(6) 0.0003(6) 0.0055(6) 0.0045(6) 0.0081(6) 0.0103(6) 0.0063(7) 0.0190(9) 0.0169(9) 0.048(2)
0.0174(5) 0.0151(4) 0.0195(5) 0.0140(6) 0.0115(5) 0.0083(5) 0.0100(5) 0.0072(6) 0.0100(6) 0.0143(7) 0.0118(8) 0.0118(9) 0.010(1) 0.0097(9) 0.0114(5) 0.0245(6) 0.0183(8) 0.0119(7) 0.0169(7) 0.0105(7) 0.0132(8) 0.0196(9) 0.0091(8) 0.019(1) 0.025(2) 0.071(3) 0.05(1) 0.0189(6) 0.0035(5) 0.0116(5) 0.0154(7) 0.0067(6) 0.0103(6) 0.0115(6) 0.0107(6) 0.0139(7) 0.0191(7) 0.0288(9) 0.0175(8) 0.036(2)
0.0197(5) 0.0247(5) 0.0237(5) 0.0170(7) 0.0194(6) 0.0150(6) 0.0190(6) 0.0175(6) 0.0129(7) 0.0278(8) 0.038(1) 0.0067(9) 0 . 0 1 1 ( 1 ) 0.0373(9) 0.0213(6) 0.0204(7) 0.0283(9) 0.0194(7) 0.0197(7) 0.0189(8) 0.0139(8) 0.0201(9) 0.026(1) 0.017(1) 0.020(1) 0.039(3) 0.026(8) 0.0383(7) 0.0180(5) 0.0133(6) 0.0188(7) 0.0148(6) 0.0157(6) 0.0177(7) 0.0196(7) 0.0222(7) 0.0260(8) 0.027(1) 0.044(1) 0.061(2)
Acknowledgment. We wish to thank the Deutsche Forschungsgemeinschaft, Bonn, for financial support.
References
1. Krämer, W.: Dimere 3-Hydroxy-o-chinone und der Mechanismus der Purpuragallinbildung. Dissertation, University of Mainz, Germany 1998.
2. Sheldrick, G. M.: SHELXL-97. Program for Crystal Structure Refine- ment. University of Göttingen, Germany 1997.
3. Altomare, Α.; Cascarano, G.; Giacovazzo, С.; Guagliardi, Α.; Burla, M.
е . ; Polidori, G.; Camalli, M.: S1R92 - a program for automatic solution of crystal structures by direct methods. J. Appi. Crystallogr. 27 (1994) 435-436.
4. Svenson, C.: CELSIUS. Program for Refinement of Lattice Parameters.
Lund, Sweden 1974.
5. Dräger, M.; Gattow G.: Kristallographische Computerprogramme für die CDC-3300. Acta Chem. Scand. 25 (1971) 761-762.