ζ . Kristallogr. NCS 213 (1998) 4 7 5
© by R. Oldenbourg Verlag, München
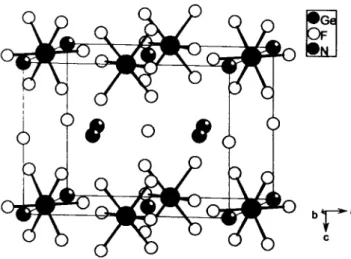
Crystal structure of triammonium heptafluorogermanate, (NH4)3GeF7
C . P l i t z k o
Uni\ ersität Hannover. Institut für Anorganische Chemie. Callinstr. 9, D-30167 Hannover. Germany a n d G . M e y e r
Uni\ ersität zu Köln. Institut für Anorganische Chemie. Greinstr. 6, D-50939 Köln. Germany Received February 4. 1998, transferred to 2nd update of database ICSD in 1998, CSD-No. 404261
F7GeHi2N3, tetragonal, PAImbm (No. 127), a =8.210(1) Â, с =5.984(1) A, V = 4 0 3 . 3 Â ^ Z = 2 , R(F) =0.055, R^n =0.140.
Table 1. Parameters used for the X-ray data collection
Crystal: colorless, irregular, size 0.1 χ 0.1 χ 0.1 mm Wavelength: Mo Ka radiation (0.71073 Â)
μ: 38.68 cm-'
Diffractometer: Stoe EPDS
Scan mode: 50 exposures, Δφ = 3°
293 К
2θιιωχ: 54.94°
275 Criterion for Л,: /ο>2σ(/ο) N(param)n;/üi«í: 23
Programs: SHELXS-86, SHELXL-93
Source of material: Colorless single crystals of (МН4)зСеР7 were obtained during the reaction of Ge powder with (NH4)HF2 (molar ratio 1:4) in a sealed Monel (CU32NÌ68) ampoule at 5 7 3 K. At higher temperature (> 6 7 3 К ), (NH4)GeF6 (see ref. 2) is the main product.
The crystal structure of triannmonium heptafluorogermanate con- tains as the i s o ^ i c (NH4)3SiF7 (see ref. 3) isolated [GePe]
octahedra [ ¿ ( G e ' ^ - F l = 175.6 pm (F2); 175.8 pm (F3)] and lonesome F- ions ( F l ) .
Table 2. Final atomic coordinates and displacement parameters (in Â^)
Atom Site X У ζ Un U22 ί/33 Un Í/13 1/23
Ge 2d 0 Ml 0 0.0433(7) Un 0.0373(8) -0.0031(6) 0 0
F(l) 2b 0 0 1/2 0.075(4) Un 0.079(7) 0 0 0
F(2) 4« 0.3488(8) x+m 0 0.195(9) Un 0.113(8) -0.13(1) 0 0
F(3) Sk 0.1008(6) x+m 0.219(1) 0.151(5) Un 0.099(4) -0.054<6) -0.041(3) Un
N(l) 2a 0 0 0 0.10(1) Un 0.18(3) 0 0 0
N(2) Ah 0.293(1) «-1/2 1/2 0.104(5) Un 0.050(5) -0.044(7) 0 0
Acknowledgment. We thank the Deutsche Forschungsgemeinschaft for fin- 2.
ancial support.
3.
References
1. Plitzko, C.: Neue komplexe Ammoniumfluoride und Fluorid-Ammonia- 4.
kate durch Umsetzung von Metallen mit Ammonium- und Hydrazinium-
fluoriden. Dissertation, Universität Hannover, Germany 1996. 5.
Vajnstejn, B. K.; Kurdjumova, R. N.: Cubic Modification of (КН4)2СеРб.
Soy. Phys. Ciyst. 3 (1958) 27-30.
Hoard, L.; Williams, M. В.: Structures of Complex Fluorides. Ammonium Hexafluorosilicate - Ammonium Huoride, (NH4)2SiF6 · NH4F. J. Am.
Chem. Soc. 64 (1942) 633-637.
Sheldrick, G. M.: Phase Annealing in SHELX-90: Direct Methods for Large Structures. Acta Crystallogr. A46 (1990) 467-473.
Sheldrick, G. M.: SHELXL-93. Program for refining crystal structures.
University of Göttingen, Germany 1993.