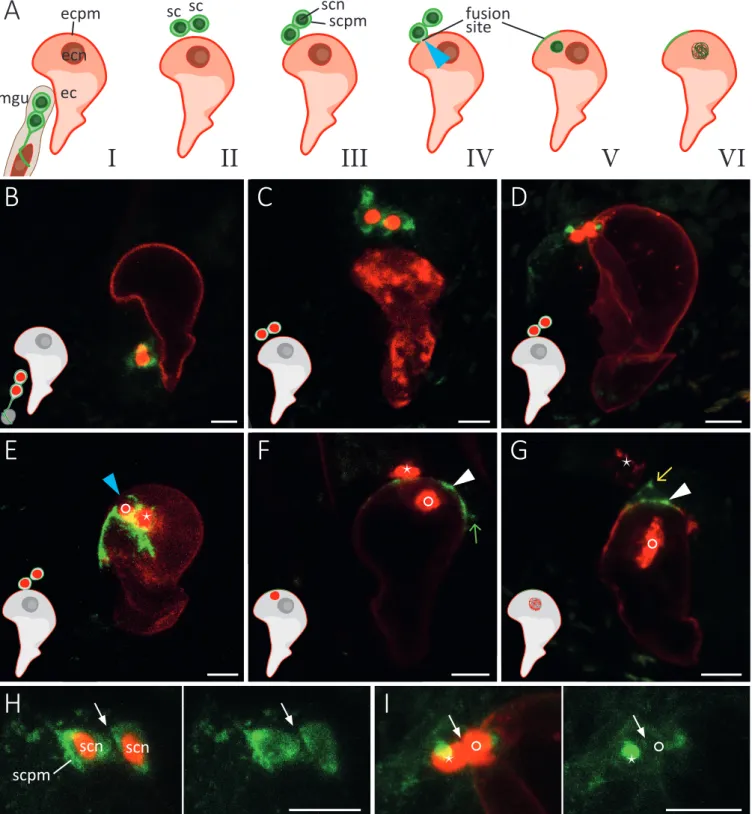
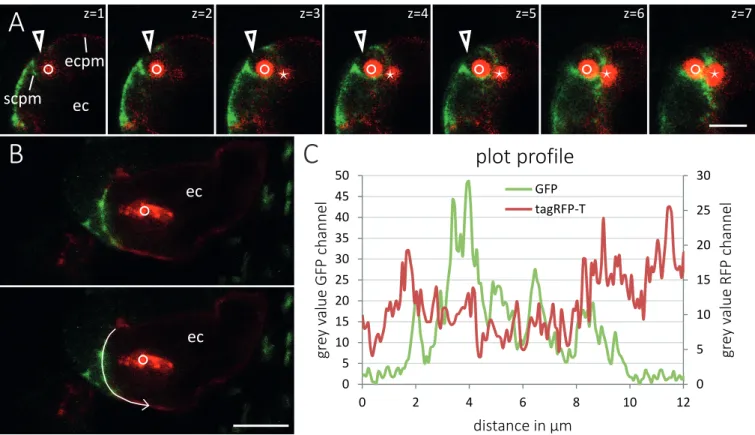
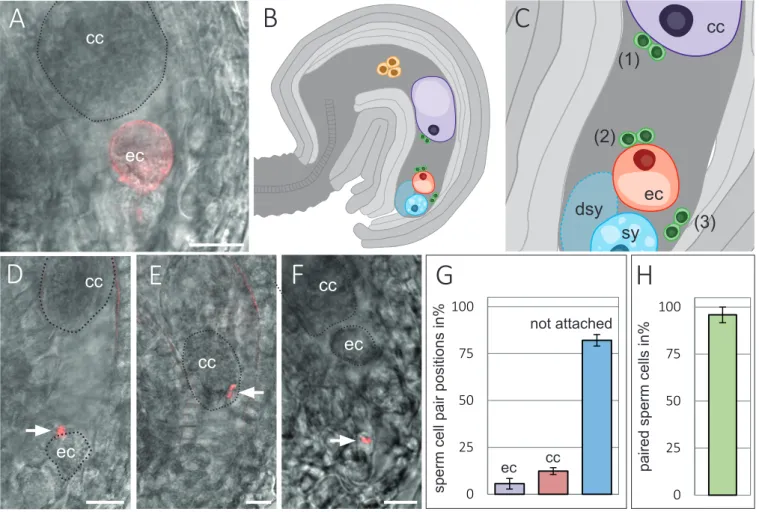
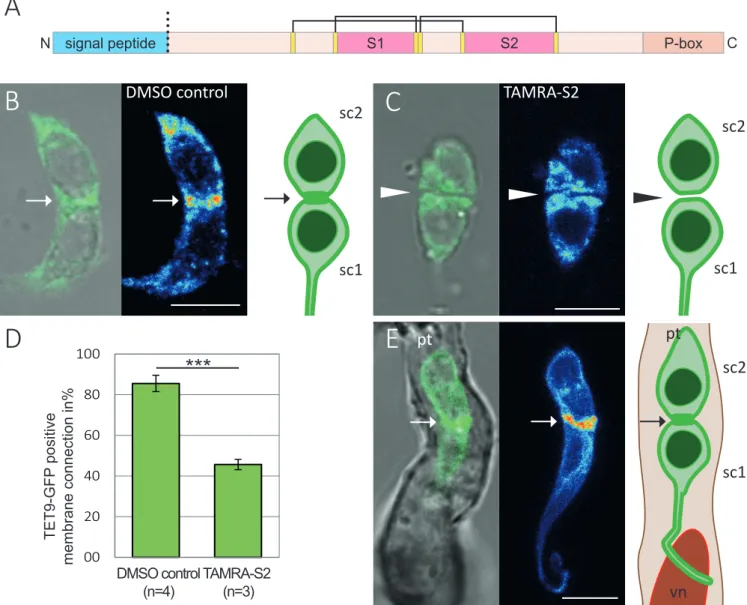
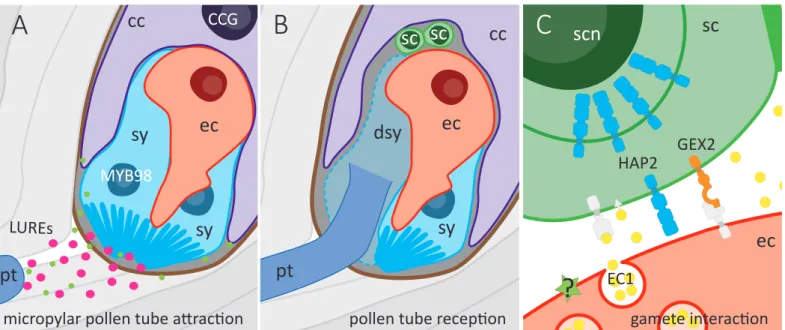
Direct gamete interactions and E
Volltext
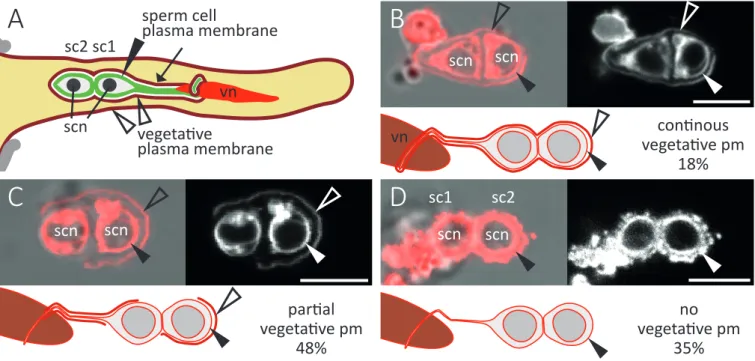
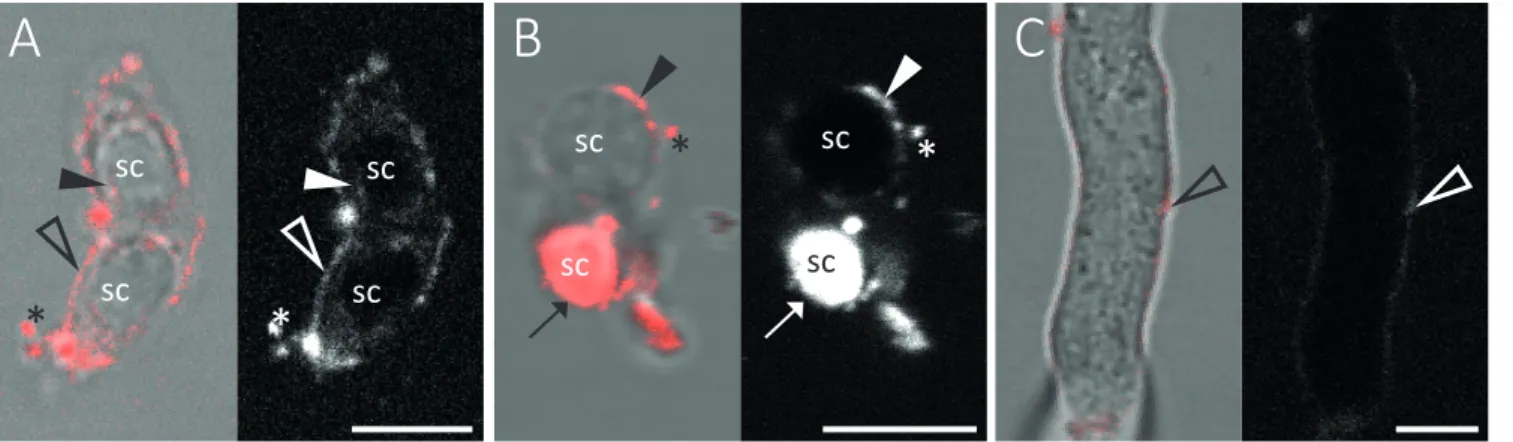
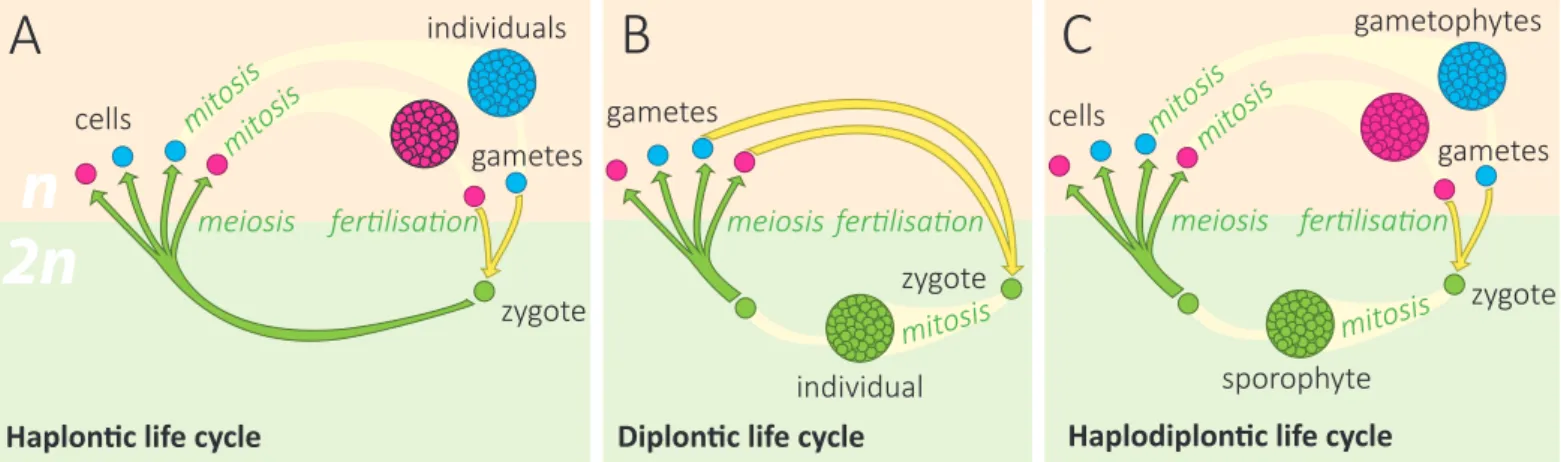
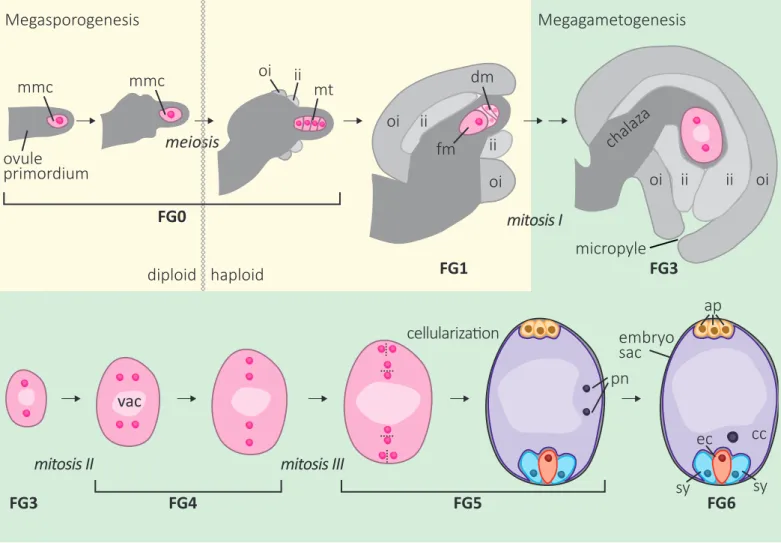
Abbildung
ÄHNLICHE DOKUMENTE
Molecular Machinery for Insertion of Tail- Anchored Membrane Proteins into the Endoplasmic Reticulum Membrane in Mammalian Cells. Sumoylated protein tyrosine phosphatase 1B
Since it was shown that PIP2 regulates membrane tension through its interaction with actin binding proteins, it might be possible that the interaction of PIP2 with MBP plays a role
mIgE-BCRs contain the evolutionarily conserved ITT motif in the mIgE cytoplasmic tail, which increases the antigen sensitivity of these BCRs via utilization of the
The geometry of the β-strands excludes that individual β-strands can exist in a lipid bilayer and all known integral membrane proteins with transmembrane β-strands form barrel
In order to compare the concentration levels found in the follicular and sperm fluids with the pattern of a commercial polychlorinated biphenyl mixture (Clo- phen A 60) and the
Hunter, Role of the membrane-spanning domain of human immunodeficiency virus type 1 envelope glycoprotein in cell-cell fusion and virus infection., J.. White, Intermonomer disulfide
Cultures co-expressing HbpD-SpT2, HbpD-SpT2-LL or HbpD-SpT2-LL with BAM were incubated with SpC2-mScarlet or not to allow for coupling of the fluorescent protein to the
(1997) A role for the disintegrin domain of cyritestin, a sperm surface protein belonging to the ADAM family, in mouse sperm-egg plasma membrane adhesion and fusion.