Journal Article
CD169+ lymph node macrophages have protective functions in mouse breast cancer metastasis
Author(s):
Tacconi, Carlotta; Commerford, Catharina D.; Dieterich, Lothar C.; Schwager, Simon; He, Yuliang;
Ikenberg, Kristian; Friebel, Ekaterina; Becher, Burkhard; Tugues, Sònia; Detmar, Michael Publication Date:
2021-04-13 Permanent Link:
https://doi.org/10.3929/ethz-b-000479427
Originally published in:
Cell Reports 35(2), http://doi.org/10.1016/j.celrep.2021.108993
Rights / License:
Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International
This page was generated automatically upon download from the ETH Zurich Research Collection. For more information please consult the Terms of use.
ETH Library
CD169 + lymph node macrophages have protective functions in mouse breast cancer metastasis
Graphical abstract
Highlights
d
Lymph node CD169
+macrophages massively proliferate and expand in murine breast cancer
d
Medullary and subcapsular sinus macrophages strongly respond to tumor stimuli
d
Depletion of subcapsular sinus macrophages strongly favors lung metastases
d
The anti-metastatic effect of CD169
+LN macrophages depends on B cells
Authors
Carlotta Tacconi,
Catharina D. Commerford,
Lothar C. Dieterich, ..., Burkhard Becher, So`nia Tugues, Michael Detmar
Correspondence
tugues@immunology.uzh.ch (S.T.), michael.detmar@pharma.ethz.ch (M.D.)
In brief
Using two distinct breast cancer models, Tacconi et al. demonstrate that CD169
+lymph node macrophages expand in tumor conditions and that their depletion from tumor-draining lymph nodes leads to an increased metastatic burden. They show that the anti-metastatic effects of CD169
+macrophages require the presence of B cells. These findings have potential clinical relevance.
Tacconi et al., 2021, Cell Reports35, 108993 April 13, 2021ª2021 The Authors.
https://doi.org/10.1016/j.celrep.2021.108993
ll
Report
CD169 + lymph node macrophages have protective functions in mouse breast cancer metastasis
Carlotta Tacconi,1,4Catharina D. Commerford,1,4Lothar C. Dieterich,1Simon Schwager,1Yuliang He,1 Kristian Ikenberg,1,2Ekaterina Friebel,3Burkhard Becher,3So`nia Tugues,3,*and Michael Detmar1,5,*
1Institute of Pharmaceutical Sciences, ETH Zurich, Zurich, Switzerland
2Institute of Pathology and Molecular Pathology, University Hospital Zurich, Zurich, Switzerland
3Department of Experimental Immunology, University of Zurich, Zurich, Switzerland
4These authors contributed equally
5Lead contact
*Correspondence:tugues@immunology.uzh.ch(S.T.),michael.detmar@pharma.ethz.ch(M.D.) https://doi.org/10.1016/j.celrep.2021.108993
SUMMARY
Although the contribution of macrophages to metastasis is widely studied in primary tumors, the involvement of macrophages in tumor-draining lymph nodes (LNs) in this process is less clear. We find CD169
+macro- phages as the predominant macrophage subtype in naive LNs, which undergo proliferative expansion in response to tumor stimuli. CD169
+LN macrophage depletion, using an anti-CSF-1R antibody or clodro- nate-loaded liposomes, leads to increased metastatic burden in two mouse breast cancer models. The expansion of CD169
+macrophages is tightly connected to B cell expansion in tumor-draining LNs, and B cell depletion abrogates the effect of CD169
+macrophage absence on metastasis, indicating that the CD169
+macrophage anti-metastatic effects require B cell presence. These results reveal a protective role of CD169
+LN macrophages in breast cancer metastasis and raise caution for the use of drugs aiming at the depletion of tumor-associated macrophages, which might simultaneously deplete macrophages in tumor-draining LNs.
INTRODUCTION
Distant organ metastasis is the major cause of death in cancer patients and due to resistance acquisition and immune evasion, traditional therapies frequently fail to cure or even treat cancer.
Tumor-associated macrophages (TAMs) have a range of tu- mor-promoting functions and are considered to represent a promising therapeutic target (Qian and Pollard, 2010). However, attempts to manipulate TAMs have yielded varying outcomes, ranging from decreased to increased tumor progression (Bona- pace et al., 2014;MacDonald et al., 2010;Palucka and Cous- sens, 2016;Pyonteck et al., 2013;Qian et al., 2011;Ries et al., 2014;Rolny et al., 2011;Swierczak et al., 2014;van den Boorn and Hartmann, 2013).
Manipulations of the myeloid system during tumor progres- sion have mainly been performed systemically, therefore ne- glecting the influence of the treatment on other macrophage populations, such as those residing in the tumor-draining lymph nodes (LNs). Three distinct macrophage subsets have been described in the LN: subcapsular sinus (SCS) macrophages (F4/80 CD169+), medullary sinus (MS) macrophages (F4/
80+CD169+), and medullary cord (MC) macrophages (F4/
80+CD169 ) (Gray and Cyster, 2012). By capturing antigen, im- mune complexes, and viruses, CD169+LN macrophages pre- vent pathogen spread (Hashimoto et al., 2011b; Iannacone et al., 2010;Kastenm€uller et al., 2012;Palucka and Coussens,
2016) and initiate immune responses (Carrasco and Batista, 2007;Junt et al., 2007;Panni et al., 2013;Phan et al., 2007;
Saunderson et al., 2014). SCS macrophages have been shown to drive B cell activation and humoral immunity (Chatziandreou et al., 2017;Gaya et al., 2015;Moalli et al., 2015;Phan et al., 2009), as well as to cross-present dead tumor cell-derived an- tigen to CD8+T cells (Asano et al., 2011). In humans, a high density of CD169+macrophages in regional LNs significantly correlates with an increased survival rate in patients with colo- rectal carcinoma (Ohnishi et al., 2013), melanoma (Saito et al., 2015), and endometrial carcinoma (Ohnishi et al., 2016), and with early clinical stages in breast cancer (Shiota et al., 2016).
Thus, although the depletion of TAMs in the primary tumor could be beneficial in many types of cancer, these studies sug- gest that a concurrent reduction of CD169+LN macrophages might be detrimental for the initiation of anti-tumor immunity.
Whether these cells could also have an impact on the spread of cancer cells to distal organs is largely unknown.
By using the murine 4T1 and the MMTV-PyMT mammary car- cinoma models, we show here that CD169+macrophages in tu- mor-draining LNs expand during disease progression and adapt to tumor-derived signals through changes in their gene expres- sion pattern. Importantly, the depletion of CD169+LN macro- phages led to increased lung metastasis in 4T1 tumor-bearing mice, which was dependent on the presence of B cells. Overall, we identify a protective role of CD169+ LN macrophages in
cancer metastasis and show that this is mediated through the activation of B cell responses.
RESULTS
Massive expansion of monocyte and macrophage populations in tumor-draining LNs
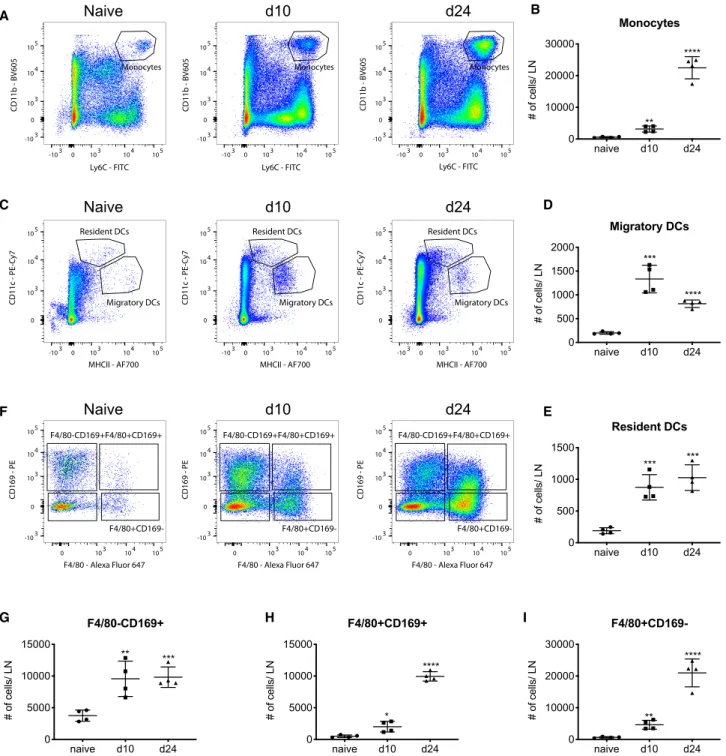
We used fluorescence-activated cell sorting (FACS) analyses of naive and 4T1 tumor-draining LNs to study LN myeloid cell sub- sets in steady state and during tumor progression. After exclu- sion of B cells and T cells (CD19 and CD3 ), we found several CD11b+myeloid populations, such as CD11b+Ly6G+neutrophils and CD11b+LY6Chimonocytes (Figure S1A). Unique to LNs, we found macrophages expressing CD169, which could be subdivided into SCS macrophages (F4/80 CD169+) and MS macrophages (F4/80+CD169+) (Figure S1A). We also identified a population of F4/80+CD169 cells, which may include mono- cyte-derived and MC macrophages (Figure S1A). By gating on major histocompatibility complex (MHC) class II+CD11c+cells, we could also distinguish between MHC class IIintCD11chiresi- dent and MHC class IIhiCD11cintmigratory dendritic cells (DCs) (Figure S1A). The quantification of these myeloid subsets showed an increase of essentially all monocyte (Figures 1A and 1B), DC (Figures 1C–1E), and macrophage (Figures 1F–1I) populations in tumor-draining LNs in cell number (Figure 1), as well as in the percent of all living cells (Figures S1B–S1G), both at early (day 10 after tumor cell injection) and late (day 24 after tumor cell injection) tumor stages. To confirm these findings, we used the MMTV-PyMT breast cancer model and found an in- crease of all macrophage populations (Figures S1K–S1M), as well as CD11b+Ly6C+monocytes (Figure S1H) and DCs (Figures S1I and S1J).
Overall, our data demonstrate an expansion of several myeloid populations in tumor-draining LNs, which led to an over-propor- tional presence of myeloid cells in both tumor models, the most prominent subtype being CD169+macrophages.
Depletion of CD169+macrophages in tumor-draining LNs leads to increased lung metastasis
We next investigated how the manipulation of the LN myeloid compartment influences tumor progression. Because survival of macrophages has been shown to depend on colony stimu- lating factor 1 receptor (CSF-1R) signaling (Hashimoto et al., 2011a;MacDonald et al., 2010), we treated 4T1 tumor-bearing mice with an anti-CSF-1R antibody or the respective control immunoglobulin G (IgG) (Figure S2A). Anti-CSF-1R treatment efficiently depleted both F4/80+and F4/80 CD169+LN macro- phages independently of tumor burden, in naive and tumor- bearing mice (Figures S2B–S2E), whereas it did not deplete CD169 macrophages (Figures S2C and S2F). CSF-1R blockade also led to a tumor-dependent increase in LN weight (Fig- ure S2G), correlating with an expansion of non-CD169+macro- phages in tumor-draining LNs (Figures S2C and S2F). Strikingly, although primary tumor growth was not changed (Figure S2H), anti-CSF-1R-treated mice showed a significant increase in the number and size of lung metastatic nodules (Figures S2I and S2J), raising the question whether CD169+LN macrophages can inhibit distant organ metastasis. In the primary tumors,
monocytes, but not F4/80+ TAMs, were also increased (Fig- ure S2K) upon CSF-1R blockade, in accordance with a previous study showing a de-differentiation into more inflammatory monocytes rather than a depletion of TAMs after blockade of CSF-1R signaling (Swierczak et al., 2014). In the lungs, a decrease of alveolar macrophages, but not of F4/80+macro- phages, was observed (Figure S2L). Although monocytes and DCs were not changed, both CD169+and F4/80+macrophages were also reduced in the spleen of anti-CSF-1R-treated mice (Figure S2M).
Because systemic anti-CSF-1R treatment has multiple effects on other myeloid cells apart from CD169+LN macrophages, we aimed at targeting CD169+macrophages in tumor-draining LNs specifically and locally. To this end, we injected clodronate- loaded liposomes (CLLs) or empty control liposomes (ctrs) into the calf of 4T1 tumor-bearing mice for drainage to the popliteal and to the tumor-draining inguinal LN (Figure 2A). In agreement with previous reports (Delemarre et al., 1990;Junt et al., 2007;
Van Rooijen and Sanders, 1994), drainage of CLLs specifically targeted CD169+LN macrophages (Figures 2B andS3A–S3E), but not any other LN populations in both naive (Figures S3A–
S3C) and tumor-bearing mice (Figures 2C, 2D, andS3D–S3G).
CLL treatment did not alter LN weight (Figure 2E), primary tumor growth (Figure 2F), or the infiltration of myeloid cells into the pri- mary tumor (Figures S3J–S3L), the lung (Figures S3M–S3P), and the spleen (Figures S3Q–S3S). However, similar to the anti-CSF- 1R treatment, local CLL treatment roughly doubled the number of metastatic nodules in the lung and led to a significant increase in average nodule size (Figure 2G). Similar results were also observed in the PyMT orthotopic mouse model of breast cancer metastasis, where CLL-loaded liposomes induced local deple- tion of CD169+LN macrophages (Figures S3T–S3V) and signifi- cantly increased lung metastatic nodules without affecting LN and tumor weight (Figures S3W–S3Y).
To exclude potential artifacts as a result of aberrant tumor im- plantation, we also injected CLLs or empty liposomes once PyMT tumors were already established (Figure 2H). Also, with this treatment regimen, CD169+macrophage depletion led to an increased lung metastatic burden without affecting LN weight and primary tumor growth (Figures 2I–2M).
We also used transgenic mice expressing the human diph- theria toxin (DT) receptor under the CD169 promoter (CD169- DTR) to specifically deplete CD169+macrophages (Figure S4A).
Upon systemic DT administration, CD169+ LN macrophages were significantly reduced in transgenic animals compared with their littermate controls (Figure S4B). This treatment did not lead to any changes in LN weight, frequencies of F4/
80+CD169 macrophages in the tumor-draining LN, or lung metastasis (Figures S4C–S4E). However, we observed a signif- icant reduction of the CD169+subset of TAMs (Figure S4F) and smaller primary tumors (Figure S4G). To achieve a more spe- cific depletion of tumor-draining LN CD169+cells, we injected DT locally into the calf (Figure S4H). To our surprise, also in these experimental conditions, we observed an important sys- temic reduction of CD169+ macrophages in both draining (inguinal) and non-draining (auricular) LNs (Figure S4I), as well as in the primary tumor (Figure S4J), spleen (Figure S4K), and lung (Figure S4L) of CD169-DTR tumor-bearing mice. Even
though LN weight was not affected (Figure S4M), there was a significant reduction of primary tumor growth (Figure S4N) and metastatic score (Figure S4O). Importantly, CD169 MC macrophages were not changed upon DTR administration indicating the specificity of the model used (Figure S4I). These findings are in contrast with local CD169+ LN macrophage
depletion studies performed so far and are most likely ex- plained by the systemic depletion of CD169+macrophages in the primary tumor and other organs that might reinforce anti-tu- mor immune response.
These data show that the local depletion of CD169+macro- phages in tumor-draining LNs leads to an increase in distant
A B
C D
F E
G H I
Figure 1. LN monocyte and macrophage subpopulations and their expansion in 4T1 tumor-draining LNs
(A–I) FACS plots from gating inFigure S1A and analysis of myeloid cell populations in the LNs of naive and 4T1 tumor-bearing mice, displayed as total number of cells per LN. All data are presented as mean±SD. Significance was determined by one-way ANOVA with Dunnett’s multiple comparison test (nR4 mice/group, representative of two experiments). p < 0.05 (*), p < 0.01 (**), p < 0.001 (***), and p < 0.0001 (****).
A
C
F
H
K
I
L M
J G
D E
B
(legend on next page)
organ metastasis without affecting primary tumor growth, sug- gesting that these cells have a protective, anti-metastatic function.
CD169+LN macrophages strongly respond to tumor stimuli
To better understand which changes LN CD169+macrophages undergo in tumor conditions, we evaluated the distribution of these cells in LNs of naive and tumor-bearing mice (Figures 3A and 3B). Despite the swelling of tumor-draining LNs, the network of CD169+ macrophages remained dense and morphologically unchanged in 4T1 tumor-draining LNs (Figures 3A and 3B). We next compared gene expression patterns of SCS macrophages (F4/80 CD169+) and MS macrophages (F4/80+CD169+) both in homeostatic and tumor-bearing condi- tions by RNA sequencing. The analysis revealed important dif- ferences between the two CD169+subsets (Figures S5A and S5B), with 714 genes upregulated by SCS macrophages (log2
fold change [log2FC] > 1 and p < 0.05) and 862 genes enriched in MS macrophages (log2FC > 1 and p < 0.05) (Table S1), which could be associated in part with both adaptive and innate im- mune-stimulatory pathways (Tables S2 and S3). Both SCS and MS macrophages from tumor-draining LNs upregulated a large number of genes associated with cell proliferation (DNA replication, chromosome condensation and segregation, cell cycle and division) (Figures 3C;Tables S2andS3). In support of these data, we found a strong co-localization of Ki67 and CD169 in tumor-draining LNs (Figure 3D). Although about 14% of SCS and 28% of MS macrophages were positive for Ki67 in the LNs under homeostatic conditions, Ki67 expression increased to over 21% and 70% in tumor-draining LN SCS and MS macrophages (Figures 3E), suggesting that the expansion of CD169+macrophages in tumor-draining LNs is at least in part due to local proliferation. We also assessed the protein levels of the co-stimulatory molecule CD80 and the mannose receptor CD206, which we found to be downregulated in tu- mor-draining LN MS macrophages (Table S1). Whereas tumor development did not affect the expression of these two markers in SCS macrophages (Figures S5C and S5D), MS macrophages in tumor-draining LNs showed decreased levels
of CD80 and decreased amounts of CD206 in comparison with those from naive mice (Figures 3F, 3G,S5C, and S5D).
This was accompanied by a significant reduction in MHC class II expression (Figures S5E and S5F).
Altogether, this suggests that, although the general morpho- logical position of both subsets of CD169+macrophages re- mains unchanged in tumor-draining LNs, they do adapt to tumor stimuli during tumor progression.
The anti-metastatic effect of CD169+LN macrophages depends on B cells
The gene expression profiles of tumor-draining LN SCS and MS macrophages showed an upregulation of processes related to B cell activation for SCS (Figure 3H), whereas MS macrophages presented a marked enrichment for genes related to antigen processing and presentation on MHC class I, hinting to T cell modulation (Figure 3J) in tumor-draining conditions. We thus hypothesized that CD169+ LN macrophages dampen lung metastasis by regulating adaptive anti-tumor immunity in the tu- mor-draining LN. First, we studied the association between the number of SCS and MS macrophages with B and T lympho- cytes, respectively. FACS analysis revealed an increase of B cells, including subsets such as germinal center B cells, B cells expressing the activation and maturity markers CD25 or CD69, and B cells expressing the memory and plasma cell marker CD27 (Figures S5G and S5H). However, although we observed a significant increase in the percentages of germinal center and CD69+B cells in tumor-draining conditions, the frequencies of CD25+and CD27+B cells over the total B cells were signifi- cantly decreased in tumor-bearing mice compared with empty liposomes controls (Figure S5I). We did not observe differences in the number of T cells (Figure S5H). Thus, only the number of B cells closely correlated with the number of SCSs in the LN but was negatively associated with lung metastatic burden (Figures 3I, 3L, and 3M). Similarly, we observed a strong negative corre- lation between SCS and MS macrophages with lung metasta- ses (Figures 3I, 3L, and 3N). In sharp contrast, there was no cor- relation between the number of T cells and MS macrophages (Figure 3K), and T cell abundance did not influence metastatic burden (Figure 3O). Moreover, local depletion of CD169+LN
Figure 2. Local clodronate treatment causes depletion of CD169+LN macrophages and increases lung metastasis
(A) Schematic schedule for the treatment with clodronate-loaded liposomes (CLLs) or empty control liposomes (ctrs) in 4T1 tumor-bearing mice.
(B) FACS data showing the number of SCS and MS CD169+macrophages per tumor-draining LN in mice treated with ctrs or CLLs (n = 8 mice/group, repre- sentative of two individual experiments).
(C and D) FACS data showing the number of total F4/80+CD169 macrophages and CD11b+F4/80 monocytes per tumor-draining LN in mice treated with ctrs or CLLs (n = 8 mice/group, representative of two individual experiments).
(E) Tumor-draining LN weight in mice treated with ctrs or CLLs (n = 8 mice/group, representative of two individual experiments).
(F)In vivotumor volume as calculated from caliper measurements and tumor weight determinedex vivo(n = 10 mice/group, representative of two individual experiments).
(G) Representative images of India ink-stained lungs from both groups with arrowheads indicating metastatic nodules. Quantification of the number and average size of metastatic nodules per lung (data pooled from two individual experiments with a total of n = 14 mice/group).
(H) Schematic schedule for the treatment with ctrs or CLLs in PyMT tumor-bearing mice once tumors are already established.
(I and J) FACS data showing the number of SCS, MS CD169+, and MC CD169 macrophages per tumor-draining LN in mice treated with ctrs or CLLs (nR6 mice/
group).
(K) Tumor-draining and non-draining LN weight in mice treated with ctrs or CLLs (n = 6 mice/group, representative of two individual experiments).
(L) Tumor weight determinedex vivo(nR6 mice/group).
(M) Quantification of the number and average size of metastatic nodules per lung after India ink staining of lungs (R6 mice/group). All data are presented as mean±SD. Significance was determined by two-way ANOVA (tumor volume) or unpaired Student’s t test. p < 0.01 (**) and p < 0.0001 (****).
MB, macrophage.
A B
D
F
H
J
I
K
L M N O
E C
G
Figure 3. CD169+LN macrophages are profoundly affected by tumor-derived stimuli
(A and B) CD169+macrophages in the SCS and MS of naive (A) and tumor-draining (B, 20 days after inoculation) LNs. Confocal images of naive and tumor-draining inguinal LNs stained for LYVE-1 and CD169. Scale bars: 200mm. The boxed areas in the left panels are shown at higher magnification on the right. Scale bars: 25mm.
(C) Gene set enrichment analysis (GSEA) enrichment plots of gene sets correlated to cell division enriched in both SCS (left panel) and MS (right panel) mac- rophages from tumor-draining LNs compared with naive LNs.
(legend continued on next page)
macrophages using CLLs led to a reduction of B cells, but not T cells, in tumor-draining LNs of both 4T1 and PyMT tumor- bearing mice (Figures 4A and 4B), which was even seen when CD169+ LN macrophages were depleted after tumors were already established (Figure 4C). To exclude a direct effect of CLLs on B cells, we FACS-sorted B and T cells from LNs of naive mice and exposed them to medium only, empty lipo- somes, or CLLs and cultured them for 18 h, using the BV2 macrophage mouse line as a positive control. As expected, CLLs induced cell death of BV2 cells without affecting the viability of both B and T cells (Figure S5J). Moreover, naive mice locally treated with empty liposomes and contralaterally with CLLs displayed significantly reduced CD169+LN macro- phages, leaving B cells unchanged (Figure S5K).
Reduction of B cells, but not T cells, upon CLL treatment was observed also in blood (Figure 4D), tumors (Figure 4E), and lungs (Figure 4F) of 4T1-bearing mice, suggesting that the expansion of B cells depends on the presence of this particular macro- phage population. Moreover, we did not find significant changes in the levels of putative B regulatory cells (CD25+CD69+) in the blood, lung, or draining LNs (Figure S5L).
Fittingly, RNA sequencing of SCS macrophages from tumor- draining LNs showed the upregulation of several genes that are implicated in presenting antigen to B cells (Figure S5M). Of these, Fc gamma receptor 1 (FcgR1), tumor necrosis factor re- ceptor superfamily, member 4 (Tnfrsf4), leukocyte immunoglob- ulin like receptor B3 (Pirb), and others (Figure S5M) were signif- icantly upregulated in SCS macrophages of tumor-draining LNs.
To test whether B cells were required for the anti-metastatic activity of SCS macrophages, we used an anti-CD20 antibody (a-CD20) to deplete B cells or the respective IgG control (IgG) in 4T1 tumor-bearing mice that were either locally depleted for CD169+LN macrophages (CLLs) or not (ctrs) (Figures 4G–4I).
Neither of these treatments had an effect on primary tumor growth (Figure 4J). Strikingly, the increased lung metastatic load observed upon CD169+ LN macrophage depletion was dependent on the presence of B cells because it was abol- ished in mice that were systemically depleted of B cells (Figure 4K).
These results indicate that CD169+ macrophages mediate their anti-metastatic effect via the regulation of B cells.
DISCUSSION
An increasing number of reports have shown that myeloid cells facilitate primary tumor invasion and metastasis (Mantovani et al., 2002;Qian and Pollard, 2010), but little is known about the involvement of myeloid cells localized in tumor-draining
LNs, the major site for the initiation of immune responses and frequently affected by metastasis (Gray and Cyster, 2012;Na- thanson et al., 2015). Here we show in two independent breast cancer models that LNs indeed remodel their myeloid compart- ment in response to tumor stimuli, similar to what was observed at the primary tumor site (Movahedi et al., 2010). CD169+macro- phages expanded over the course of tumor progression, allow- ing for the maintenance of a dense macrophage network in the SCS. Although this contrasts with the disrupted SCS macro- phage barrier previously described in LNs draining melanomas (Pucci et al., 2016), infection, or inflammation (Chatziandreou et al., 2017;Gaya et al., 2015), we propose that the local prolifer- ation observed in CD169+LN macrophages may account for the maintenance of the SCS macrophage network. The ability of other tissue-resident macrophages to proliferate locally has been reported previously (Hashimoto et al., 2013; Mondor et al., 2019).
To date, most preclinical studies targeting TAMs used sys- temic therapies, sometimes leading to conflicting results for metastatic models. Anti-CCL2 treatment inhibited monocyte recruitment to tumor beds and lung metastasis in breast cancer (Lu and Kang, 2009;Qian et al., 2011), whereas discontinuance of the treatment caused accelerated metastasis and death (Bo- napace et al., 2014). Likewise, anti-CSF-1R treatment reduced tumor burden in colorectal adenocarcinoma, fibrosarcoma (Ries et al., 2014), and glioma (Pyonteck et al., 2013), but not in breast cancer, where it led to increased metastatic disease (Swierczak et al., 2014). In accordance, we also found increased lung metastasis in anti-CSF-1R-treated 4T1 tumor- bearing mice. We propose that this is caused by the depletion of CD169+LN macrophages because we observed the same metastatic outcome when this population was specifically abla- ted upon local CLL administration. However, the systems used to deplete CD169+LN macrophages in this study, although very efficient, might have also affected the phenotype or function- ality of other phagocytic cells. Thus, we cannot fully discard the contribution of other myeloid subsets to the pro-metastatic effects observed here. In an attempt to specifically target LN CD169+ macrophages, we took advantage of CD169-DTR mice; however, despite local injection, DT treatment ablated CD169+cells systemically in the primary tumor and other or- gans, leading to a reduction of primary tumor growth that compromised the analysis of metastasis in CD169-DTR mice.
We thus achieved a more specific depletion of CD169+LN mac- rophages using a local delivery of clodronate. Because multiple anti-CSF-1/CSF-1R compounds are in clinical trials for different solid tumor types (Hamilton et al., 2016), our findings are of spe- cial importance and suggest that a careful monitoring of LN
(D) Confocal image of the CD169+macrophages of a day 20 4T1 tumor-draining LN stained for CD169 and the proliferation marker Ki67. Scale bar: 25mm.
(E–G) FACS analysis of Ki67, CD80, and CD206 expression in SCS and MS macrophages from naive and 4T1 tumor-draining LNs. Quantification shows per- centage of positive cells out of all SCS and MS macrophages. All data are presented as mean±SD. Significance was determined by unpaired Student’s t test (nR4 mice/group). p < 0.05 (*) and p < 0.0001 (****).
(H and J) GSEA enrichment plots of gene sets enriched in SCS (H) and MS (J) macrophage-derived tumor-draining compared with naive LNs.
(I and K) Correlation between CD169+macrophages and B cell subsets or T cells, respectively, quantified by FACS in LNs of naive and tumor-bearing mice.
(L–O) Correlation between the number of SCS macrophages (L), B cells (M), MSM macrophages (N), or T cells (O), as assessed by FACS, and lung metastatic load, as assessed byin vivoimaging system (IVIS) measurement of luciferase+4T1 tumor cells (average radiance per lung) (n = 5 naive and 10 tumor-bearing mice, representative of two experiments).
A
B
C
E
G
K
D
F
H I
J
(legend on next page)
macrophages could support a rational and successful design for these therapies. Whether other treatments that modulate TAMs, such as chemotherapy and radiation (De Palma and Lewis, 2013; Mantovani and Allavena, 2015), also influence CD169+ macrophages in tumor-draining LNs remains to be elucidated.
To better understand the possible interaction of SCS and MS macrophages with T and B cells, we characterized these two cell subtypes by RNA sequencing of cells isolated from naive and tu- mor-draining LNs. Both SCS and MS macrophage transcrip- tome profiles were profoundly altered upon tumor challenge and highlighted two possible mechanisms contributing to tumor metastasis. SCS macrophages from tumor-bearing mice upre- gulated a set of genes involved in B cell-induced immunity, while MS macrophages were enriched in genes supporting T cell acti- vation. Although T cells did not correlate with CD169+LN macro- phages or with metastatic burden, we found that the abundance of SCS macrophages was associated with the expansion and activation of B cells, which appear to be key mediators of the anti-metastatic effects exerted by CD169+ LN macrophages.
Indeed, the poorly endocytic capacity of CD169+SCS macro- phages makes them optimal candidates for the presentation of unprocessed antigen to B cells (Phan et al., 2009), and there is ample evidence for the involvement of CD169+LN macrophages in B cell activation in response to particulate antigen, such as im- mune complexes or viral particles (Carrasco and Batista, 2007;
Chatziandreou et al., 2017;Junt et al., 2007;Phan et al., 2007, 2009). Reports about the involvement of CD169+LN macro- phages in carcinogenesis, however, are conflicting. Whereas CD169+LN macrophages were reported to mediate the trans- port of tumor antigen into B cell follicles (Moalli et al., 2015), others reported that they prevent the induction of B cell-medi- ated pro-tumorigenic immunity by blocking antigen access to the LN (Pucci et al., 2016).
Nevertheless, neither of these studies considered the occur- rence of metastasis in their experimental settings, which we found to depend on the protective function of SCS macrophages through regulation of B cells. Similar toPucci et al. (2016), we
observed a significant increase of CD169+LN macrophages in tumor-draining conditions. However, although they reported an acceleration in melanoma tumor growth associated with increased B cell density upon CD169+LN macrophage deple- tion, we did not observe a difference in primary tumor size in any of the tumor models analyzed but did observe a decreased B cell abundance in tumor-draining LNs, primary tumors, and lungs, suggesting different immune mechanisms occurring in melanoma and breast cancer.
B regulatory cells (defined as CD25+CD69+B cells) have been reported to promote breast cancer metastasis (Olkhanud et al., 2011). We did not find differences in this putative B regulatory cell population upon CLL treatment; therefore, more investiga- tion is needed to delineate the specific role of B cells in this met- astatic model.
We found that systemic B cell depletion did not mimic the local ablation of CD169+macrophages, suggesting a more complex function of B cells in metastasis. Indeed, B cells have been re- ported to have either pro- or anti-tumorigenic functions, depend- ing on their phenotype and the stage of tumor progression (Tsou et al., 2016;Yuen et al., 2016). For instance, B cells have been shown to be essential for de novo carcinogenesis following chronic inflammation (de Visser et al., 2005) or to adopt a regula- tory phenotype, contributing to the suppression of T cell immunity (Inoue et al., 2006). However, B cells can enhance cytotoxic T cell activity, have direct tumoricidal effects, and indirectly contribute to tumor cell killing by antibody-dependent mechanisms (Tsou et al., 2016). It is thus possible that the depletion of CD169+LN macrophages leads to a specific reduction of anti-tumor B cells, thus enhancing lung metastases. Importantly, the anti-tumor ac- tivity of B cells has also been described in the 4T1 breast cancer model (Bodogai et al., 2013;Xia et al., 2016), thus confirming the protective role of these cells in this cancer type. Also, a link be- tween the presence of B cells and a favorable prognosis has been shown in human breast cancer patients (Iglesia et al., 2014;Mahmoud et al., 2012;Schmidt et al., 2008). Along those lines, it would be of interest to study how CD169+macrophages regulate B cell-mediated immune responses in other lymphoid
Figure 4. B cells are required for the anti-metastatic activity of SCS macrophages
(A) FACS analysis of CD19+B220+(B cells) and T cell subsets in the LNs of 4T1 tumor-bearing mice treated with empty ctrs or CLLs, pre-gated on single living CD45+cells defined as CD3+CD8+CD4 (CD8 T cells), CD3+CD8 CD4+Foxp3 (CD4 conventional T cells), and CD3+CD8 CD4+CD25+Foxp3+(T regulatory cells) displayed as percentage of all single living cells in the LN (n = 8 mice/group).
(B) FACS analysis of B cell and T cell subsets in the LNs of PyMT tumor-bearing mice treated with ctrs or CLLs, pre-gated on single living cells defined as CD19+B220+(B cells), CD3+CD8+CD4 (CD8 T cells), CD3+CD8 CD4+Foxp3 (CD4 conventional T cells), and CD3+CD8 CD4+CD25+Foxp3+(T regulatory cells) displayed as percentage of all single living cells in the LN (nR6 mice/group).
(C) FACS analysis of B cells in the LNs of PyMT tumor-bearing mice treated with empty ctrs or CLLs 5 days after tumor cell injection, pre-gated on single living cells defined as CD19+B220+(B cells) displayed as percentage of all single living cells in the LN (nR6 mice/group).
(D) FACS analysis of B (CD19+B220+), plasma (B220+CD138+), and T (CD3+) cells, pre-gated on single living CD45+cells of 4T1 tumor-bearing mice treated with ctrs or CLLs expressed as percentage of all living cells in blood (n = 8 mice/group).
(E and F) FACS analysis of B (CD19+B220+) and T (CD3+) cells of 4T1 tumor-bearing mice treated with ctrs or CLLs expressed as percentage of CD45+cells in tumor (E) and lung (F).
(G) Schematic schedule for the treatment with CLLs and anti-CD20 antibody (a-CD20) or their respective controls (ctrs and IgG) in 4T1 tumor mice.
(H and I) FACS data shown as the number of CD169+macrophages or B cells expressed as percentage of living cells per tumor-draining LN in mice treated with CLL and/or a-CD20 and/or their respective controls.
(J)In vivotumor volume as calculated from caliper measurements and tumor weight as determinedex vivo.
(K) Representative images of India ink-stained lungs from all four groups with arrowheads indicating metastatic nodules and quantification of the number and average size of metastatic nodules per lung (n = 10 mice/group).
All data are presented as mean±SD. Significance was determined by unpaired Student’s t test (G) and by two-way ANOVA. p < 0.05 (*) and p < 0.001 (***). MB, macrophage.
tissues, such as tertiary lymphoid structures (TLSs). TLSs are transient ectopic lymphoid aggregates structurally and function- ally similar to LNs (Colbeck et al., 2017;Kratz et al., 1996), and their presence in certain types of cancers, including breast can- cer, is correlated with better prognosis (Hiraoka et al., 2016;
Saute`s-Fridman et al., 2016,2019;Willis et al., 2009).
Collectively, our data demonstrate a protective, anti-metastatic function of CD169+ SCS macrophages during carcinogenesis, mediated by the activation and expansion of B cells in tumor-drain- ing LNs. Based on our results and recent studies reporting that high CD169+LN macrophage density correlates with a better clin- ical prognosis or early clinical stage in patients with colorectal car- cinoma, malignant melanoma, endometrial carcinoma, and breast cancer (Komohara et al., 2017;Shiota et al., 2016), we thus pro- pose that CD169+LN macrophages act as important sentinels capable of mounting anti-tumor immune responses and should therefore be taken into account for anti-metastatic therapies.
STAR+METHODS
Detailed methods are provided in the online version of this paper and include the following:
d KEY RESOURCES TABLE
d RESOURCE AVAILABILITY B Lead contact
B Materials availability B Data and code availability
d EXPERIMENTAL MODEL AND SUBJECT DETAILS B Mice
B Cell lines
d METHOD DETAILS B Tumor models
B Macrophage and B cell depletion B Detection of lung metastasis
B FACS sorting and FACS analysis of LNs and tumors B Clodronate liposomes cytotoxicity assay
B RNA extraction and sequencing of CD169+ LN macro- phages
B Immunofluorescence staining of murine LN sections
d QUANTIFICATION AND STATISTICAL ANALYSIS
SUPPLEMENTAL INFORMATION
Supplemental information can be found online athttps://doi.org/10.1016/j.
celrep.2021.108993.
ACKNOWLEDGMENTS
We thank Prof. Cornelia Halin (ETH Zurich) for valuable input and Jeannette Scholl, Jasmin Frey, Carlos Ochoa Pereira (all ETH Zurich), Catharine Aquino, and Dr. Emilio Ya´ng€uez (both FGCZ Zurich) for excellent technical assistance.
This study was supported by Swiss National Science Foundation grants 166490 and 185392, European Research Council grant LYVICAM, the Sassella Foundation, and the Promedica Foundation.
AUTHOR CONTRIBUTIONS
Conceptualization, C.T., C.D.C., S.T., and M.D.; methodology, C.T., C.D.C., S.T., and L.C.D.; validation, C.T. and C.D.C.; formal analysis, C.T., C.D.C.,
Y.H., and E.F.; investigation, C.D.C., C.T., L.C.D., K.I., and S.S.; resources, B.B. and M.D.; writing – original draft, C.T., C.D.C., S.T., and M.D.; writing – review & editing, C.T., C.D.C., L.C.D., K.I., Y.H., S.S., E.F., S.T., and M.D.; su- pervision, S.T. and M.D.; project administration, C.T., C.D.C., S.T., and M.D.;
funding acquisition, M.D.
DECLARATION OF INTERESTS
The authors declare no competing interests.
Received: May 14, 2020 Revised: November 1, 2020 Accepted: March 24, 2021 Published: April 13, 2021 REFERENCES
Asano, K., Nabeyama, A., Miyake, Y., Qiu, C.H., Kurita, A., Tomura, M., Kana- gawa, O., Fujii, S., and Tanaka, M. (2011). CD169-positive macrophages domi- nate antitumor immunity by crosspresenting dead cell-associated antigens.
Immunity34, 85–95.
Bodogai, M., Lee Chang, C., Wejksza, K., Lai, J., Merino, M., Wersto, R.P., Gress, R.E., Chan, A.C., Hesdorffer, C., and Biragyn, A. (2013). Anti-CD20 anti- body promotes cancer escape via enrichment of tumor-evoked regulatory B cells expressing low levels of CD20 and CD137L. Cancer Res.73, 2127–2138.
Bolger, A.M., Lohse, M., and Usadel, B. (2014). Trimmomatic: a flexible trimmer for Illumina sequence data. Bioinformatics30, 2114–2120.
Bonapace, L., Coissieux, M.M., Wyckoff, J., Mertz, K.D., Varga, Z., Junt, T., and Bentires-Alj, M. (2014). Cessation of CCL2 inhibition accelerates breast cancer metastasis by promoting angiogenesis. Nature515, 130–133.
Carrasco, Y.R., and Batista, F.D. (2007). B cells acquire particulate antigen in a macrophage-rich area at the boundary between the follicle and the subcapsu- lar sinus of the lymph node. Immunity27, 160–171.
Chatziandreou, N., Farsakoglu, Y., Palomino-Segura, M., D’Antuono, R., Piz- zagalli, D.U., Sallusto, F., Lukacs-Kornek, V., Uguccioni, M., Corti, D., Turley, S.J., et al. (2017). Macrophage Death following Influenza Vaccination Initiates the Inflammatory Response that Promotes Dendritic Cell Function in the Drain- ing Lymph Node. Cell Rep.18, 2427–2440.
Colbeck, E.J., Ager, A., Gallimore, A., and Jones, G.W. (2017). Tertiary Lymphoid Structures in Cancer: Drivers of Antitumor Immunity, Immunosup- pression, or Bystander Sentinels in Disease? Front. Immunol.8, 1830.
De Palma, M., and Lewis, C.E. (2013). Macrophage regulation of tumor re- sponses to anticancer therapies. Cancer Cell23, 277–286.
de Visser, K.E., Korets, L.V., and Coussens, L.M. (2005). De novo carcinogen- esis promoted by chronic inflammation is B lymphocyte dependent. Cancer Cell7, 411–423.
Delemarre, F.G., Kors, N., Kraal, G., and van Rooijen, N. (1990). Repopulation of macrophages in popliteal lymph nodes of mice after liposome-mediated depletion. J. Leukoc. Biol.47, 251–257.
Dobin, A., Davis, C.A., Schlesinger, F., Drenkow, J., Zaleski, C., Jha, S., Batut, P., Chaisson, M., and Gingeras, T.R. (2013). STAR: ultrafast universal RNA-seq aligner. Bioinformatics29, 15–21.
Gaya, M., Castello, A., Montaner, B., Rogers, N., Reis e Sousa, C., Bruckba- uer, A., and Batista, F.D. (2015). Host response. Inflammation-induced disruption of SCS macrophages impairs B cell responses to secondary infec- tion. Science347, 667–672.
Gray, E.E., and Cyster, J.G. (2012). Lymph node macrophages. J. Innate Immun.4, 424–436.
Hamilton, J.A., Cook, A.D., and Tak, P.P. (2016). Anti-colony-stimulating factor therapies for inflammatory and autoimmune diseases. Nat. Rev. Drug Discov.
16, 53–70.
Hashimoto, D., Chow, A., Greter, M., Saenger, Y., Kwan, W.H., Leboeuf, M., Ginhoux, F., Ochando, J.C., Kunisaki, Y., van Rooijen, N., et al. (2011a). Pre- transplant CSF-1 therapy expands recipient macrophages and ameliorates
GVHD after allogeneic hematopoietic cell transplantation. J. Exp. Med.208, 1069–1082.
Hashimoto, D., Miller, J., and Merad, M. (2011b). Dendritic cell and macro- phage heterogeneity in vivo. Immunity35, 323–335.
Hashimoto, D., Chow, A., Noizat, C., Teo, P., Beasley, M.B., Leboeuf, M., Becker, C.D., See, P., Price, J., Lucas, D., et al. (2013). Tissue-resident mac- rophages self-maintain locally throughout adult life with minimal contribution from circulating monocytes. Immunity38, 792–804.
Hiraoka, N., Ino, Y., and Yamazaki-Itoh, R. (2016). Tertiary Lymphoid Organs in Cancer Tissues. Front. Immunol.7, 244.
Iannacone, M., Moseman, E.A., Tonti, E., Bosurgi, L., Junt, T., Henrickson, S.E., Whelan, S.P., Guidotti, L.G., and von Andrian, U.H. (2010). Subcapsular sinus macrophages prevent CNS invasion on peripheral infection with a neuro- tropic virus. Nature465, 1079–1083.
Iglesia, M.D., Vincent, B.G., Parker, J.S., Hoadley, K.A., Carey, L.A., Perou, C.M., and Serody, J.S. (2014). Prognostic B-cell signatures using mRNA-seq in patients with subtype-specific breast and ovarian cancer. Clin. Cancer Res.20, 3818–3829.
Inoue, S., Leitner, W.W., Golding, B., and Scott, D. (2006). Inhibitory effects of B cells on antitumor immunity. Cancer Res.66, 7741–7747.
Junt, T., Moseman, E.A., Iannacone, M., Massberg, S., Lang, P.A., Boes, M., Fink, K., Henrickson, S.E., Shayakhmetov, D.M., Di Paolo, N.C., et al. (2007).
Subcapsular sinus macrophages in lymph nodes clear lymph-borne viruses and present them to antiviral B cells. Nature450, 110–114.
Kastenm€uller, W., Torabi-Parizi, P., Subramanian, N., La¨mmermann, T., and Germain, R.N. (2012). A spatially-organized multicellular innate immune response in lymph nodes limits systemic pathogen spread. Cell150, 1235–
1248.
Komohara, Y., Ohnishi, K., and Takeya, M. (2017). Possible functions of CD169-positive sinus macrophages in lymph nodes in anti-tumor immune re- sponses. Cancer Sci.108, 290–295.
Kratz, A., Campos-Neto, A., Hanson, M.S., and Ruddle, N.H. (1996). Chronic inflammation caused by lymphotoxin is lymphoid neogenesis. J. Exp. Med.
183, 1461–1472.
Liao, Y., Smyth, G.K., and Shi, W. (2019). The R package Rsubread is easier, faster, cheaper and better for alignment and quantification of RNA sequencing reads. Nucleic Acids Res.47, e47.
Love, M.I., Huber, W., and Anders, S. (2014). Moderated estimation of fold change and dispersion for RNA-seq data with DESeq2. Genome Biol.15, 550.
Lu, X., and Kang, Y. (2009). Chemokine (C-C motif) ligand 2 engages CCR2+
stromal cells of monocytic origin to promote breast cancer metastasis to lung and bone. J. Biol. Chem.284, 29087–29096.
MacDonald, K.P., Palmer, J.S., Cronau, S., Seppanen, E., Olver, S., Raffelt, N.C., Kuns, R., Pettit, A.R., Clouston, A., Wainwright, B., et al. (2010). An anti- body against the colony-stimulating factor 1 receptor depletes the resident subset of monocytes and tissue- and tumor-associated macrophages but does not inhibit inflammation. Blood116, 3955–3963.
Mahmoud, S.M.A., Lee, A.H.S., Paish, E.C., Macmillan, R.D., Ellis, I.O., and Green, A.R. (2012). The prognostic significance of B lymphocytes in invasive carcinoma of the breast. Breast Cancer Res. Treat.132, 545–553.
Mantovani, A., and Allavena, P. (2015). The interaction of anticancer therapies with tumor-associated macrophages. J. Exp. Med.212, 435–445.
Mantovani, A., Sozzani, S., Locati, M., Allavena, P., and Sica, A. (2002). Macro- phage polarization: tumor-associated macrophages as a paradigm for polar- ized M2 mononuclear phagocytes. Trends Immunol.23, 549–555.
Moalli, F., Proulx, S.T., Schwendener, R., Detmar, M., Schlapbach, C., and Stein, J.V. (2015). Intravital and whole-organ imaging reveals capture of mela- noma-derived antigen by lymph node subcapsular macrophages leading to widespread deposition on follicular dendritic cells. Front. Immunol.6, 114.
Mondor, I., Baratin, M., Lagueyrie, M., Saro, L., Henri, S., Gentek, R., Suerinck, D., Kastenmuller, W., Jiang, J.X., and Baje´noff, M. (2019). Lymphatic Endothe- lial Cells Are Essential Components of the Subcapsular Sinus Macrophage Niche. Immunity50, 1453–1466.e4.
Movahedi, K., Laoui, D., Gysemans, C., Baeten, M., Stange´, G., Van den Bos- sche, J., Mack, M., Pipeleers, D., In’t Veld, P., De Baetselier, P., and Van Gin- derachter, J.A. (2010). Different tumor microenvironments contain functionally distinct subsets of macrophages derived from Ly6C(high) monocytes. Cancer Res.70, 5728–5739.
Nathanson, S.D., Shah, R., and Rosso, K. (2015). Sentinel lymph node metas- tases in cancer: causes, detection and their role in disease progression.
Semin. Cell Dev. Biol.38, 106–116.
Ohnishi, K., Komohara, Y., Saito, Y., Miyamoto, Y., Watanabe, M., Baba, H., and Takeya, M. (2013). CD169-positive macrophages in regional lymph nodes are associated with a favorable prognosis in patients with colorectal carci- noma. Cancer Sci.104, 1237–1244.
Ohnishi, K., Yamaguchi, M., Erdenebaatar, C., Saito, F., Tashiro, H., Katabu- chi, H., Takeya, M., and Komohara, Y. (2016). Prognostic significance of CD169-positive lymph node sinus macrophages in patients with endometrial carcinoma. Cancer Sci.107, 846–852.
Olkhanud, P.B., Damdinsuren, B., Bodogai, M., Gress, R.E., Sen, R., Wejksza, K., Malchinkhuu, E., Wersto, R.P., and Biragyn, A. (2011). Tumor-evoked reg- ulatory B cells promote breast cancer metastasis by converting resting CD4+ T cells to T-regulatory cells. Cancer Res.71, 3505–3515.
Palucka, A.K., and Coussens, L.M. (2016). The Basis of Oncoimmunology. Cell 164, 1233–1247.
Panni, R.Z., Linehan, D.C., and DeNardo, D.G. (2013). Targeting tumor-infil- trating macrophages to combat cancer. Immunotherapy5, 1075–1087.
Phan, T.G., Grigorova, I., Okada, T., and Cyster, J.G. (2007). Subcapsular encounter and complement-dependent transport of immune complexes by lymph node B cells. Nat. Immunol.8, 992–1000.
Phan, T.G., Green, J.A., Gray, E.E., Xu, Y., and Cyster, J.G. (2009). Immune complex relay by subcapsular sinus macrophages and noncognate B cells drives antibody affinity maturation. Nat. Immunol.10, 786–793.
Pucci, F., Garris, C., Lai, C.P., Newton, A., Pfirschke, C., Engblom, C., Alvarez, D., Sprachman, M., Evavold, C., Magnuson, A., et al. (2016). SCS macro- phages suppress melanoma by restricting tumor-derived vesicle-B cell inter- actions. Science352, 242–246.
Pyonteck, S.M., Akkari, L., Schuhmacher, A.J., Bowman, R.L., Sevenich, L., Quail, D.F., Olson, O.C., Quick, M.L., Huse, J.T., Teijeiro, V., et al. (2013).
CSF-1R inhibition alters macrophage polarization and blocks glioma progres- sion. Nat. Med.19, 1264–1272.
Qian, B.Z., and Pollard, J.W. (2010). Macrophage diversity enhances tumor progression and metastasis. Cell141, 39–51.
Qian, B.Z., Li, J., Zhang, H., Kitamura, T., Zhang, J., Campion, L.R., Kaiser, E.A., Snyder, L.A., and Pollard, J.W. (2011). CCL2 recruits inflammatory mono- cytes to facilitate breast-tumour metastasis. Nature475, 222–225.
Ries, C.H., Cannarile, M.A., Hoves, S., Benz, J., Wartha, K., Runza, V., Rey- Giraud, F., Pradel, L.P., Feuerhake, F., Klaman, I., et al. (2014). Targeting tu- mor-associated macrophages with anti-CSF-1R antibody reveals a strategy for cancer therapy. Cancer Cell25, 846–859.
Rolny, C., Mazzone, M., Tugues, S., Laoui, D., Johansson, I., Coulon, C., Squa- drito, M.L., Segura, I., Li, X., Knevels, E., et al. (2011). HRG inhibits tumor growth and metastasis by inducing macrophage polarization and vessel normalization through downregulation of PlGF. Cancer Cell19, 31–44.
Saito, Y., Ohnishi, K., Miyashita, A., Nakahara, S., Fujiwara, Y., Horlad, H., Mo- toshima, T., Fukushima, S., Jinnin, M., Ihn, H., et al. (2015). Prognostic Signif- icance of CD169+ Lymph Node Sinus Macrophages in Patients with Malignant Melanoma. Cancer Immunol. Res.3, 1356–1363.
Saunderson, S.C., Dunn, A.C., Crocker, P.R., and McLellan, A.D. (2014).
CD169 mediates the capture of exosomes in spleen and lymph node. Blood 123, 208–216.
Saute`s-Fridman, C., Lawand, M., Giraldo, N.A., Kaplon, H., Germain, C., Frid- man, W.H., and Dieu-Nosjean, M.C. (2016). Tertiary Lymphoid Structures in Cancers: Prognostic Value, Regulation, and Manipulation for Therapeutic Intervention. Front. Immunol.7, 407.
Saute`s-Fridman, C., Petitprez, F., Calderaro, J., and Fridman, W.H. (2019).
Tertiary lymphoid structures in the era of cancer immunotherapy. Nat. Rev.
Cancer19, 307–325.
Schmidt, M., Bo¨hm, D., von To¨rne, C., Steiner, E., Puhl, A., Pilch, H., Lehr, H.A., Hengstler, J.G., Ko¨lbl, H., and Gehrmann, M. (2008). The humoral immune sys- tem has a key prognostic impact in node-negative breast cancer. Cancer Res.
68, 5405–5413.
Shiota, T., Miyasato, Y., Ohnishi, K., Yamamoto-Ibusuki, M., Yamamoto, Y., Iwase, H., Takeya, M., and Komohara, Y. (2016). The Clinical Significance of CD169-Positive Lymph Node Macrophage in Patients with Breast Cancer.
PLoS ONE11, e0166680.
Subramanian, A., Tamayo, P., Mootha, V.K., Mukherjee, S., Ebert, B.L., Gil- lette, M.A., Paulovich, A., Pomeroy, S.L., Golub, T.R., Lander, E.S., and Me- sirov, J.P. (2005). Gene set enrichment analysis: a knowledge-based approach for interpreting genome-wide expression profiles. Proc. Natl. Acad. Sci. USA 102, 15545–15550.
Swierczak, A., Cook, A.D., Lenzo, J.C., Restall, C.M., Doherty, J.P., Anderson, R.L., and Hamilton, J.A. (2014). The promotion of breast cancer metastasis caused by inhibition of CSF-1R/CSF-1 signaling is blocked by targeting the G-CSF receptor. Cancer Immunol. Res.2, 765–776.
Tsou, P., Katayama, H., Ostrin, E.J., and Hanash, S.M. (2016). The Emerging Role of B Cells in Tumor Immunity. Cancer Res.76, 5597–5601.
van den Boorn, J.G., and Hartmann, G. (2013). Turning tumors into vaccines:
co-opting the innate immune system. Immunity39, 27–37.
Van Rooijen, N., and Sanders, A. (1994). Liposome mediated depletion of mac- rophages: mechanism of action, preparation of liposomes and applications.
J. Immunol. Methods174, 83–93.
Willis, S.N., Mallozzi, S.S., Rodig, S.J., Cronk, K.M., McArdel, S.L., Caron, T., Pinkus, G.S., Lovato, L., Shampain, K.L., Anderson, D.E., et al. (2009). The microenvironment of germ cell tumors harbors a prominent antigen-driven hu- moral response. J. Immunol.182, 3310–3317.
Xia, Y., Tao, H., Hu, Y., Chen, Q., Chen, X., Xia, L., Zhou, L., Wang, Y., Bao, Y., Huang, S., et al. (2016). IL-2 augments the therapeutic efficacy of adoptively transferred B cells which directly kill tumor cells via the CXCR4/CXCL12 and perforin pathways. Oncotarget7, 60461–60474.
Yuen, G.J., Demissie, E., and Pillai, S. (2016). B lymphocytes and cancer: a love-hate relationship. Trends Cancer2, 747–757.
Zeisberger, S.M., Odermatt, B., Marty, C., Zehnder-Fja¨llman, A.H., Ballmer-Hofer, K., and Schwendener, R.A. (2006). Clodronate-lipo- some-mediated depletion of tumour-associated macrophages: a new and highly effective antiangiogenic therapy approach. Br. J. Cancer 95, 272–281.
STAR + METHODS
KEY RESOURCES TABLE
REAGENT or RESOURCE SOURCE IDENTIFIER
Antibodies
Rat anti-CSF1R This paper AFS98
Rat IgG Sigma Aldrich Cat# I4131; RRID:AB_1163627
Rat anti-CD20 Genentech clone 5D2
Fc block BioLegend Cat# 101302; RRID:AB_312801
CD45.2-APC-Cy7 BioLegend Cat# 103116; RRID:AB_312981
CD45-PB BioLegend Cat# 103126; RRID:AB_493535
CD169-PE BioLegend Cat# 142403; RRID:AB_10915470
CD169-PE-Cy7 BioLegend Cat# 142412; RRID:AB_2563911
CD11c-PE-Cy7 BioLegend Cat# 117318; RRID:AB_493568
CD11c-PerCP-Cy5 BioLegend Cat# 117328; RRID:AB_2129641
Ly6G-PerCP-Cy5.5 BD Cat# 560602; RRID:AB_1727563
Ly6G-PB BD Cat# 560603; RRID:AB_1727564
Ly6G-FITC BD Cat# 551460; RRID:AB_394207
F4/80-AF647 BioRad Cat# MCA497A647; RRID:AB_323931
F4/80-Biotin BioRad Cat# MCA497B; RRID:AB_322046
Ly6C-FITC BD Cat# 553104; RRID:AB_394628
Ly6C-APC-Cy7 BD Cat# 560596; RRID:AB_1727555
CD11b-BV605 BioLegend Cat# 101237; RRID:AB_11126744
CD11b-PerCP-Cy5.5 BioLegend Cat# 101228; RRID:AB_893232
CD11b-FITC BioLegend Cat# 101206; RRID:AB_312789
MHCII-AF700 BioLegend Cat# 107622; RRID:AB_493727
CD206-PE BioLegend Cat# 141706; RRID:AB_10895754
CD80-BV650 BioLegend Cat# 104732; RRID:AB_2686972
SiglecF-BV421 BD Cat# 562681; RRID:AB_2722581
CD19-FITC BD Cat# 557398; RRID:AB_396681
B220-APC BioLegend Cat# 103212; RRID:AB_312997
GL-7-PB BioLegend Cat# 14461; RRID:AB_2563291
CD27-PE BioLegend Cat# 124210; RRID:AB_1236459
CD138-PerCP-Cy5.5 BioLegend Cat# 142509; RRID:AB_2561600
CD69-PE-Cy7 BD Cat# 552879; RRID:AB_394508
CD25-BV605 BioLegend Cat# 102035; RRID:AB_11126977
CD3-PE-Cy7 BioLegend Cat# 100320; RRID:AB_312685
CD3-FITC BioLegend Cat# 100203; RRID:AB_312660
CD4-PerCP BioLegend Cat# 100432; RRID:AB_893323
CD4-PE BD Cat# 553049; RRID:AB_394585
CD8-FITC BioLegend Cat# 100706; RRID:AB_312745
Ki67-eFluor450 eBioscience Cat# 48-5698-80; RRID:AB_11151155
Foxp3-PE-eFluor610 eBioscience Cat# 61-5773-82; RRID:AB_2574624
Rabbit anti-LYVE-1 AngioBio Cat# 11-034; RRID:AB_2813732
Goat anti-LYVE-1 R&D Cat# AF2125; RRID:AB_2297188
Rat anti-CD169 BioRad Cat# MCA884; RRID:AB_322416
Rat anti-CD68 Abcam Cat# ab53444; RRID:AB_869007
Ki67 Agilent Cat# M7249; RRID:AB_2250503
(Continued on next page)
RESOURCE AVAILABILITY Lead contact
Further information and requests for resources and reagents should be directed to and will be fulfilled by the lead contact, Michael Detmar (michael.detmar@pharma.ethz.ch).
Materials availability
This study did not generate new unique reagents.
Data and code availability
RNA sequencing data have been deposited and are accessible in ArrayExpress under accession number E-MTAB-8482.
EXPERIMENTAL MODEL AND SUBJECT DETAILS Mice
Female Balb/cByJRj (referred to as BALB/c) mice and female C57BL/6JRj (referred to as C57BL/6) mice were purchased from Janvier. CD169-DTR mice (B6.129-Siglec1 < tm1(HBEGF)Mtka > ) were imported from the RIKEN BioResource Center (Japan) Continued
REAGENT or RESOURCE SOURCE IDENTIFIER
CD169-APC BioLegend Cat# 142417; RRID:AB_2565640
Chemicals, peptides, and recombinant proteins clodronate-loaded liposomes or empty control liposomes
FormuMax Cat# F70101C-NC
luciferin Caliper LifeSciences Cat# #119222
collagenase IV GIBCO Cat# 17104019
DNase I Roche Cat# 10104159001
PharmLyse buffer BD Cat# 555899
Critical commercial assays
Zombie NIR Fixable Viability Kit BioLegend Cat# 423106
Zombie Aqua Fixable Viability Kit BioLegend Cat# 423102
RNeasy Plus Micro kit QIAGEN Cat# 74034
MycoScope Genlantis Cat# MYO1100
intracellular staining kit BioLegend Cat# 72-5775
Diphtheria Toxin Calbiochem Cat# 322326
Deposited data
RNA sequencing This paper ArrayExpress: E-MTAB-8482
Experimental models: Cell lines
4T1-luc2 Caliper Life Sciences 124087, RRID:CVCL_L899
4T1-mCherry Dr. Nicola Aceto N/A
MMTV-PyMT Dr. David DeNardo N/A
BV2 Dr. Michele Mazzanti ICLC ATL03001
AFS98 Hybridoma Dr. Richard Stanley N/A
Experimental models: Organisms/strains
Balb/cByJRj wildtype mice Janvier N/A
C57BL/6JRj wildtype mice Janvier N/A
B6.129-Siglec1 < tm1(HBEGF)Mtka > RIKEN BioResource Center RBRC04395 Software and algorithms
FlowJo TreeStar Inc https://www.flowjo.com/
Graph Pad Prism 8 Graph Pad Software https://www.graphpad.com/
R studio Bioconductor https://www.r-project.org/