Research Collection
Doctoral Thesis
Factors affecting vocal learning performance In juvenile songbirds
Author(s):
Lee, Juneseung Publication Date:
2020-02
Permanent Link:
https://doi.org/10.3929/ethz-b-000408636
Rights / License:
In Copyright - Non-Commercial Use Permitted
This page was generated automatically upon download from the ETH Zurich Research Collection. For more information please consult the Terms of use.
ETH Library
DISS. ETH NO. 26437
Factors affecting vocal learning performance In juvenile songbirds
A thesis submitted to attain the degree of DOCTOR OF SCIENCES of ETH ZURICH (Dr. sc. ETH Zurich)
presented by JUNESEUNG LEE
Dr. sc. ETH Zurich
born on 05.02.1986 citizen of Republic of Korea
accepted on the recommendation of Prof. Dr. Richard Hahnloser
Prof. Dr. Satoshi Kojima Prof. Dr. Ryosuke Tachibana
2020
Factors affecting vocal learning performance in juvenile songbirds
PhD Thesis
Juneseung Lee- Intitute of Neuroinformatics Uni / ETH Zurich
2020
Supervisor: Prof. Dr. Richard Hahnloser
Co-Supervisor: Prof. Dr. Satoshi Kojima Co-Supervisor: Prof. Dr. Ryosuke Tachibana
Abstract
Children develop language through listening and imitating vocal sounds from parents or other adult members. Similarly, songbirds, like humans, gradually acquire acoustically complex but stereotyped imitations of vocalizations produced by conspecifics. Therefore, the songbird is the ideal model to understand the underlying mechanism for vocal learning, and its study outcome can be compared with human speech learning. The songbird brain contains a premotor area HVC which is functionally equivalent to Broca’s area in humans.
HVC plays an important role in song production and perception. In both humans and songbirds, it is often found that some are good learners and others are not. It is not clear that this variability is due to diverse practical learning efforts, or just a congenital talent, or even individual differences in neural development in premotor brain areas. Our goal is to study latent mechanisms of vocal learning processes, both on the behavioral and neural levels.
One idea is that songbirds become better singers if they intensely train their vocal skills from a young age. However, we found out that in songbirds, deliberate practice does not always correlate well with the corresponding change in the imitation accuracy of their song models. In the first part of this PhD thesis, we show that the manner in which songbirds modulate acoustic variability may explain the subsequent change in performance better than the amount of practice.
Using the zebra finch as our animal model, we analyze the relationship between daily vocal practice (duration of putative singing) in juveniles and the change in acoustic similarity with their tutors’ songs. We found that there is little to no correlation between the two.
In a second part of the Thesis, we dive into in-depth neural level discovery beyond the behavioral factors for vocal learning. We hypothesize that during the vocal learning period in juvenile zebra finches there is a difference in neural activity in motor cortical areas between good and bad vocal learners. To test this hypothesis, we need to longitudinally record neural activity in HVC during the sensory and the sensory-motor period. Firstly, we show that longitudinal neural recordings can be performed in 45 dph young birds using both single- and two- photon microscopy. Then we preview the vocal learning performance of juveniles that underwent longitudinal calcium imaging. We show that some of the juveniles were still able to learn from tutoring performed during calcium imaging. In this way, we could lay a cornerstone to develop a method to understand the entire song learning process at the population level with near single-cell or single-cell resolution.
Zusammenfassung
Kinder entwickeln Sprache durch Zuhören und Imitieren von Stimmgeräuschen von Eltern oder anderen erwachsenen Mitgliedern. Ebenso erwerben Singvögel wie Menschen nach und nach akustisch komplexe, aber stereotype Imitationen von Lautäußerungen, die von Artgenossen produziert werden. Daher ist der Singvogel das ideale Modell, um den zugrundeliegenden Mechanismus für das Vokallernen zu verstehen, und sein Studienergebnis kann für das Erlernen menschlicher Sprache geteilt werden. Songbird hat eine Prämotorfläche HVC, die funktionell der Fläche von Broca beim Menschen entspricht. HVC spielt eine wichtige Rolle bei der Produktion und Wahrnehmung von Songs. Bei beiden Arten wird häufig festgestellt, dass einige gute Lernende sind und andere nicht. Es ist nicht klar, dass dies auf den unterschiedlichen praktischen Lernaufwand oder nur auf ein angeborenes Talent oder sogar auf individuelle Unterschiede in der neuralen Entwicklung im Bereich des Vormotors zurückzuführen ist. Wir möchten den verborgenen Mechanismus sowohl des Lernprozesses im Verhalten als auch des neuronalen Prozesses beim vokalen Lernen kennen.
Einige glauben, dass sie bessere Sänger hätten sein können, wenn sie ihre stimmlichen Fähigkeiten absichtlich trainiert hätten, seit sie jung waren. Wir haben jedoch herausgefunden, dass bei Singvögeln bewusstes Üben nicht immer gut mit der entsprechenden Änderung der Imitationsgenauigkeit ihrer Songmodelle korreliert. Im ersten Teil dieser Doktorarbeit haben wir gezeigt, dass die Art und Weise, wie Singvögel die akustische Variabilität modulieren, die Änderung der Leistung besser erklären kann als die Menge an Übung. Anhand des Zebrafinken als Tiermodell analysieren wir den Zusammenhang zwischen der täglichen Stimmpraxis (Dauer des mutmaßlichen Gesangs) bei Jugendlichen und der Veränderung der akustischen Ähnlichkeit mit den Liedern ihrer Tutoren. Wir fanden heraus, dass es zwischen den beiden kaum eine bis gar keine Korrelation gibt.
In einem weiteren Bereich beschäftigen wir uns mit der eingehenden Entdeckung der neuronalen Ebene, die über die Verhaltensfaktoren für das vokale Lernen hinausgeht. Wir gehen davon aus, dass es in motorisch-kortikalen Bereichen einen Unterschied in der neuronalen Aktivität zwischen guten und schlechten Stimmlernern während der Stimmlernphase beim juvenilen Zebrafinken gibt. Um die Annahme zu beweisen, müssen wir die neuronale Aktivität in der HVC während der sensorischen und sensorisch-motorischen Periode des juvenilen Zebrafinken in Längsrichtung aufzeichnen. Im zweiten Teil dieser Arbeit haben wir zum einen gezeigt, dass longitudinale neuronale Aufzeichnungen von 45dph- Jungvögeln sowohl mit einem Einzel- als auch mit einem Zweiphotonenmikroskop durchgeführt werden können. Anschließend sehen wir uns die stimmliche Lernleistung von Jugendlichen mit Längsschnitt-Calciumbildern an. Wir haben gezeigt, dass einige der Jugendlichen immer noch in der Lage sind, aus dem während der Kalziumbildgebung durchgeführten Nachhilfeunterricht zu lernen.
Auf diese Weise könnten wir den Grundstein für die Entwicklung einer Methode legen, mit der der gesamte Song-Lernprozess auf Bevölkerungsebene mit einer Auflösung von nahezu einer Zelle oder einer Zelle verstanden werden kann.
Acknowledgement
I would like to express my sincere gratitude to Prof. Richard Hahnloser for his dedicated grounding, immense knowledge and continuous support during my doctoral course. His guidance helped me in every way of research and writing of this thesis.I would also like to thank his support of the SNF grant 31003A-156976.
My doctoral course would not have completed without it. Besides my advisor, I would also like to give thanks to the rest of my thesis committee: Prof. Satoshi Kojima and Prof. Ryosuke Tachibana, for their encouragement. Even at hardship, your insights and suggestions had been the most helpful to expand my research in various perspectives.
My sincere thanks also go to Dr. Gagan Narula as my closest colleague who contributed so much on my work. I would also like to thank Dr. Joshua Herbst for providing the data for analysis. In addition, I will always remember my beloved colleagues at the songbird-group, Heiko Hörster, Ziqian Hwang, Sophie Cave- Lopez, Daniel Düring, Corinna Lorenz, Diana Rodriguez, Homare Yamahachi, and Anja Zai.
Last but not least, a special thanks to my family. Words cannot express how grateful I am to my parents and my wife for their endless love and support. Thank you so much for believing in me.
Table of Contents
Factors affecting vocal learning performance in juvenile songbirds ... 2
1. Introduction ... 8
1.1. About Songbirds and Vocal learning ... 10
1.1.1. The Zebra Finch - taeniopygia guttata ... 10
1.1.2. Birdsong - Learned Vocalizations ... 12
1.1.3. A Songbirds Brain ... 15
1.1.4. The Cortical Premotor Area HVC ... 19
2. Song imitation performance in juvenile songbirds is uncorrelated with amount of practice ... 26
2.1. Method ... 27
2.1.1. Experimental strategy ... 27
2.1.2. Tutoring ... 27
2.1.3. Song recordings ... 28
2.1.4. Song Density ... 29
2.1.5. Quantification of song development ... 29
2.2. Results ... 31
2.3. Discussion ... 40
3. Neural dynamics during vocal learning in juvenile ... 41
3.1. Introduction ... 41
3.1.1. Functional Single- and Two-Photon Imaging in Neuroscience ... 41
3.1.2. Single- and Two-Photon Microscopy ... 43
3.1.3. Fundamentals of Calcium Imaging ... 46
3.1.4. Calcium Imaging in the Zebra Finch ... 53
3.2. Methods ... 54
3.2.1. Virus injection ... 54
3.2.2. Tracer injection ... 56
3.2.3. Head plate and cranial window implantation ... 56
3.2.4. Miniscope implantation in juvenile zebra finch ... 57
3.2.5. Calcium imaging ... 58
3.2.6. Sound recording and tutor song presentation ... 58
3.3. Data analysis ... 59
3.3.1. Song similarity ... 59
3.3.2. Single and two photon imaging ... 59
3.3.3. Statistical analysis for identified neurons. ... 60
3.4. Results ... 61
3.4.1. Longitudinal quality control of cranial window ... 61
3.4.2. Longitudinal neural imaging ... 62
3.4.3. Tutoring during head-fixation ... 64
3.5. Discussion ... 65
4. Genetically encoded calcium indicators evaluated in the zebra finch ... 67
4.1. Introduction ... 67
4.2. Method ... 67
4.3. Results ... 68
4.4. Discussion ... 69
5. Welfare of zebra finches during two-photon imaging is investigated ... 70
5.1. Introduction ... 70
5.2. Method ... 71
5.2.1.Preparations for safe Head-fixation ... 71
5.2.2.Lighting in two-photon chamber without damaging to photon detector ... 71
5.2.3.Optimizing imaging duration for the well-being of subject ... 72
5.2.4.Daily condition check by body mass and no. of vocalization measuring ... 73
5.3. Discussion ... 74
Appendix A. ... 76
Bibliography ... 79
1. Introduction
One of the guiding principles for learning complex motor skills is captured by the idiom “practice makes perfect”. Watson (Watson, 1929) believed that practice is the major driving force behind success in skillful activities such as sports and music. Later, the influential work of Ericsson and colleagues (Ericsson et al., 1993b) established that deliberate practice, i.e., intense rehearsal of domain specific activity, explains much of the difference between the performance levels of athletes.
This and related work (Allen & Barnsley, 1993) led to Malcolm Gladwell’s famous
“10,000 hour” rule (Gladwell, 2009) which posits that a person needs to rehearse an activity for at least 10,000 hours to become an expert. However, recent meta- analysis has shown that the amount of deliberate practice accounts for only 20% of the variance in performance in a wide variety of motor skills, and even in cognitively demanding tasks related to education (Gobet & Campitelli, 2007;
Hambrick, Altmann, Oswald, Meinz, & Gobet, 2014; Macnamara, Hambrick, &
Oswald, 2014). The share of variance explained by practice is even lower (~1%) when only elite athletes are studied (Macnamara, Moreau, & Hambrick, 2016).
Furthermore, Macnamara et al. (2016) provide evidence for the influence on performance by factors such as the complexity of a game environment, whether it is a team/individual sport, ball vs non-ball sport, etc. These co-variates explain a significant share of the variance in performance than practice alone.
The relationship between practice and performance of a complex, natural motor behavior is of particular significance in young, maturing subjects for two reasons.
First, animals need to devote vital time and energy to practice even though the fruit of their labor might be earned in the future. Second, practice should lead to robust (stable) learning that generalizes to changing environments. To ensure robustness and strong generalization, an animal must explore many behavioral variants, which requires even more rehearsal. More concretely, if practice is considered a form of policy search for motor control (Peshkin, Kim, Meuleau, & Kaelbling, 2000; J.
Peters & Schaal, 2008; Shute, Graf, & Hansen, 2006), practice will yield better policies. Such notions are well motivated by the mathematical formulation of reinforcement learning, which is considered a prime candidate for the acquisition of skills through practice (Ericsson, 2009; Ericsson et al., 1993a; Helton, 2005;
Kulasegaram, Grierson, & Norman, 2013) in animals and in robots (policy search survey(Deisenroth, Neumann, & Peters, 2013) Jober, Peters, Diesenroth). For example, Policy gradient RL ((J. Peters & Schaal, 2008; Sutton & Barto, 1998;
Williams, 1992) is an algorithm that adapts the policy parameters to maximize
expected reward, and to compute a low-variance estimate of expected reward, the agent must perform as many rehearsals as possible.
The role of practice in animals is far from resolved because of the scarcity of densely sampled data on motor learning. We inspect the relationship between practice and performance in passerine birds, in which we have gathered longitudinal auditory recordings. Songbirds such as the zebra finch (Taeniopygia guttata) belong to a small group of species that are vocal learners, i.e. they acquire their species-specific acoustic vocabulary through sensory experience and motor practice after birth. Vocal learning begins with relatively incoherent motor
“babbling”, and slowly matures into spectro-temporally stereotyped acoustic sequences as the bird ages. The developmental process in songbirds mirrors that of human speech acquisition, although on a much shorter time scale (Allison J. Doupe
& Kuhl, 1999; Menyhart, Kolodny, Goldstein, DeVoogd, & Edelman, 2015;
Mooney, 2014).
Using the zebra finch as our animal model, we analyze the relationship between daily vocal practice (duration of putative singing) in juveniles and the change in acoustic similarity with their tutors’ songs. We find that there is little to no correlation between the two.
To go beyond behavioral comprehension, recordings of coordinated activity of neuronal populations in motor cortical areas are required during vocal development (Leonardo & Fee, 2005; Lynch, Okubo, Hanuschkin, Hahnloser, & Fee, 2016;
Okubo, Mackevicius, Payne, Lynch, & Fee, 2015a; Picardo et al., 2016a;
Simonyan & Horwitz, 2011). Recent studies during vocal learning ( Kosche et al., 2015; Okubo et al., 2015; Vallentin et al., 2015; Roberts et al., 2010) indicate that HVC’s neuronal dynamics is modulated by the tutor song. Unfortunately, neural activity in HVC has been characterized in various animals at distinct phases in different times, ranging from a few minutes to a few days. Since song learning takes at least 60 days, the previous studies lack the period required to understand the whole process. In the second part of this thesis, we established fundamental prerequisites to determine how the neuronal subpopulations (e.g. HVC neurons projecting to other nuclei) in HVC are modulated during the entire learning period.
Ultimately, we want to understand the neural modulation by auditory inputs, such as tutor song, and its neural correlation to the bird’s own song development.
In the introduction, we will layout the general knowledge on songbird and its vocal learning scheme, especially in the zebra finch. Then discuss why this is the suitable
model to study the important factors affecting to vocal learning. This PhD thesis contributes to discovering critical factors related to vocal development in both behavioral and neural system.
1.1. About Songbirds and Vocal learning
’Songbird (also called Oscine, from Latin oscen)’ is the common name of a bird belonging to the kindom Passeri of the perching birds (Passeriformes). According to Scott and Harshman, there are more than 5000 species found all over the world (Scott and Harshman, 2013). The vocal organ, syrinx, of songbirds share a common architecture and uniquely developed to produce an elaborate and diverse bird song.
Among the well-known three distantly related avian vocal learning groups – songbirds, parrots, and hummingbirds, ‘birdsong’ exclusively refers to the term of the vocal output of songbirds. In many aspects, the process of the birdsong learning of songbirds is analogous to the process of the language learning of human beings.
According to the reviews from Jarvis in 2012, vocal pathways in songbird’s brain seem to have analogous pathways implicated in human speech learning and production (Jarvis, 2012). As it is yet far way to understand human speech learning, studies in songbird brain has been implicated important neural mechanisms underlying vocal learning and production.
1.1.1. The Zebra Finch - taeniopygia guttata
Zebra finches are found natively in Austrailia (castanotis) and the East Timor and the Sunda Islands of Indonesia (guttata) (Immelmann, 1965b). They reside in almost entire continent of Australia except south and north coastal regions to avoid cool moist atmosphere. Nowadays, one can easily find them in the wildlife of the American continent or Portugal due to the human introduction (Birdlife international 2013). As they are highly social birds, wild zebra finches use to congregate a group of a few hundred individuals. Throughout their entire life, they maintain a monogamous pair relationship (Zann, 1994).
Figure 1.1.: Zebra finch family in our colony. Photograph taken by Heiko Hörster.
Zebra finches in captivity can breed all year long when sufficient water is provided and it attempts to bear young several times in each breeding season. Nottebohm showed the Juvenile zebra finches can successfully imitate the given relatively short tutor song exposure (40 playbacks of 30 seconds long) during their sensitive vocal learning period (O Tchernichovski, Lints, Mitra, & Nottebohm, 1999). Due to these reasons, zebra finches are greeted among neuroscientists studying vocal learning. Desmond Morris primarily suggested that zebra finches could be studied as an ideal behavioral model in the lab in the mid 20th century (Morris, 1954). Even though Desmond Morris’ interest in zebra finches were mainly behavioral aspects of breeding and courtship, shortly zebra finches were recognized as the most prominent animal model for neurobiology of birdsong research. Over last many decades, profound investing-ation on zebra finch brain has been performed and brought deep understanding of vocal learning and production from neural pathways to neural network level. As the vocal learning of zebra finch is analogous to the speech learning of humans, understanding of the neural mechanism underlying birdsong learning would contribute to the human’s language learning in the end (Brainard & Doupe, 2013).
1.1.2. Birdsong - Learned Vocalizations
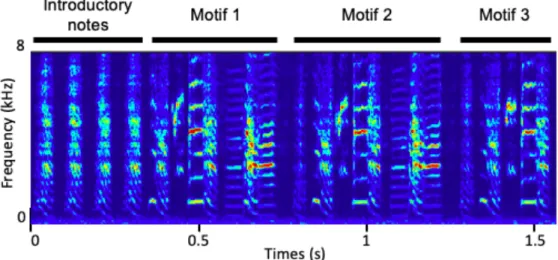
Vocal learning is a special type of motor learning shared with only few other kingdoms of animals (humans, cetaceans, bats, pinnipeds, elephants, and three bird groups – songbirds, parrots, and hummingbirds). Songbirds are distinctive vocal learners which produce complex vocalizations by learning from conspecifics. They use their singing not only to draw sexual attentions but also to defend and mark their territory (Immelmann, p. 1965a). Some songbirds sing even sing complex duets but as for zebra finches, only males produce complex learned songs and calls even though female zebra finches produce short innate calls with a wide range (Blair Simpson & Vicario, 1990). Zebra finches sing one unique and stereotyped song throughout their entire life except learning period in juvenile. Typically, their song begins with 3-5 introductory notes and a variable number of stereotyped song motif follows the notes. A song motif consists of 3-7 different syllables in robustly fixed order with 5-15 ms intermittent gap (or silence). Each syllable is about 100- 300 ms in duration and sometimes 5-50 ms sub-syllabic structures can be accompanied with it (see figure 1.2).
Figure 1.2.: Spectrogram of the song of r15s12. The color coded (from low to high power, blue to red) spectrogram shows power spectrum at different frequencies over time. This one full song bout begins with 4 introductory notes and is followed by 3-4 syllables. The 3rd rendition’s 4th syllable is truncated.
Juvenile male zebra finches can learn their song from a template provided by a conspecific male tutor bird. In general, song learning can be divided into two phases (Tamura, 1964): a sensory period during which juvenile zebra finches
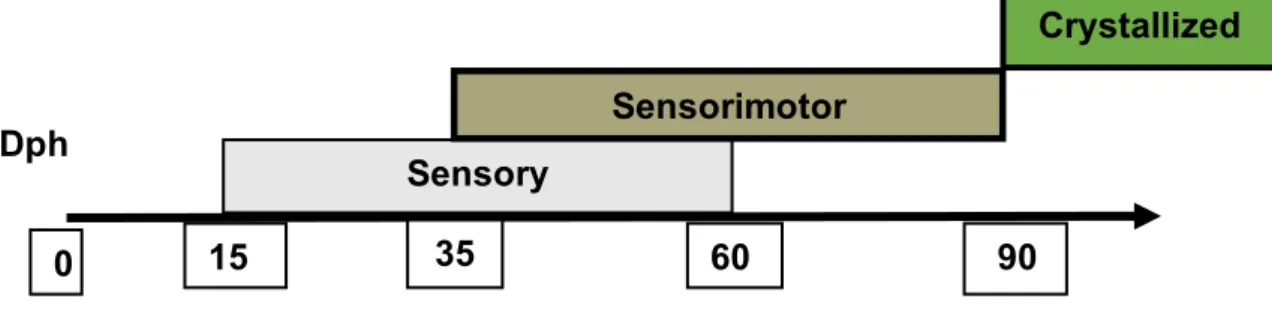
acquire a model of tutor song template and a sensorimotor period within which they refine unstructured and plastic subsong by modifying the acquired song through practice and auditory feedback. Through the two overlapping sensory and sensorimotor periods (see figure 1.3), young adult zebra finches crystallize the song at about 90 dph (Konishi, 1965).
Auditory input critically affects to the result of vocal output for juvenile zebra finches. In case of failure to hear a template song from a tutor bird at both the sensory and sensorimotor periods (typically 15-60 dph), young bird will produce abnormal and unstructured vocal output (Tamura, 1964). Konish, in 1965, found out that loss of hearing ability during practical duration can also result the same abnormal vocal output even if the young bird acquired a template from a tutor bird.
In both cases, nonetheless, its vocal output becomes more structured and stereotyped, even though it has higher plasticity than the other birds which got a template and a time to practice, after sensorimotor duration ends.
Figure 1.3.: Song learning in zebra finches undergoes in two overlapping periods: a sensory period within which a template song from a tutor male is acquired and a sensorimotor period during which vocal output is more refined to match the chosen tutor template. Normally juvenile male zebra finches start to produce very unstructured vocal output (also called subsong) at around 25 dph. Then juveniles incorporate more and more elements from the tutor song but their vocal output stays highly variable from each song bout to the next one. At the end phase of the sensorimotor period, song structure is well structured and stereotyped and this is called crystallization. Under normal conditions in nature, juvenile produces a faithful copy of the template tutor song.
If a juvenile zebra finch is exposed to a tutor song during the sensory period, it does Sensory
Sensorimotor
Crystallized Dph
0 15 35 60 90
not have to hear the template tutor song again to produce a devoted copy during practicing duration. The important factor for the faithful reproduction of the tutor song is that hearing during a sensory period for juvenile can be reproduced for the life time (Tamura, 1964). These features substantiate the theory that a process for template song memory storage during the sensory period is independent of vocal practice.
Vocal learning for juvenile songbirds is a highly precise yet rather convenient to characterize motor learning process that copies from conspecific birds. It is a straightforward example of template storage and its matching. Template matching here notes to the process of comparing the memory previously registered in sensory coordinates to the produced output sound in motor coordinates. This process needs a coordinate transformation but its mechanism has not been well understood yet.
Songbird researchers anticipate understanding of vocal learning in songbird will give a clue to the extensive comprehension of the transformation mechanism in the sensory and motor coordinates.
In further extent, the vocal learning process is analogous to human language learning. Indeed, researchers studying vocal learning suggest a set of important parallel principles in the two phenomena – human speech and birdsong: First, both birdsong and human language are learned vocalizations that serve specific communication of each species. Second, the vocalizations of birds and humans are structured by an interaction of experience and predisposition. Third, the best learning period for vocalization is both at a young age. Fourth, in both species vocal practice is followed by a duration of auditory ‘priming’. In human infants sensory priming is conducted by a loss of ability to discriminate sounds from all languages.
After sensory priming, roughly 12 months later, human babies are only able to discern between sounds they formerly learned to categorize differently. Fifth, social interaction is a critical factor to improve the learning performance in both species. Sixth, despite the fact that cortex of songbirds and mammals are differently structured, both species share distinct sets of telencephalic regions with a similar organization for vocal perception and production. The parallels even expand to the genetic level. FoxP2 is known as a gene that is not only involved in human speech related disorders but also vocal processing in songbirds (Heston & White, 2015).
Thanks to the above factors, songbirds are highlighted as a translational animal model for vocal learning to humans (Allison J. Doupe & Kuhl, 1999). In the next section, we have a close look at the brain structures and vocal pathways that provide the detail for song learning and production in songbirds.
1.1.3. A Songbirds Brain
Mammals and songbirds are vertebrates sharing a common anatomy of the central nervous system. However, telecephalon of songbirds differs from telecephalon of mammalians in the components of the pallium. The songbird’s pallium is mainly organized as unit of nuclear while the mammalian pallium is layered cortex form (Jarvis, 2004). Yet the basal ganglia in both families are nuclear organizations as part of the telencephalon.
Figure 1.4.: Schematic sagittal view of a zebra finch brain. It describes three major song related pathways: the auditory pathway (AP, green), and the motor pathway (MP, red), and the anterior forebrain pathway (AFP, blue).
Song learning and production are traditionally proclaimed to involve three major song pathways in the brain, see Figure 1.4. We briefly introduce them here:
The auditory pathway (AP) is specified by the cochlea, neural responses to auditory input, and by afferent neural connections originated from the auditory sensory organs. Since many regions of all three main pathways cross over the above criteria, the auditory pathway is normally limited to the starting of the auditory input stream, disregarding regions with known additional functionality. Moreover, neurons of the auditory pathway show responses to a wide range of auditory inputs.
Other telencephalic areas typically respond only to very specific auditory inputs.
Nucleus Ovoidalis (Ov) is the major auditory region of the thalamus (Karten, 1967). Field L is the main telencephalic region projected by the Ov (Vates,
DLM
LMAN NCM
Field L
Nif CM
Uva Ov
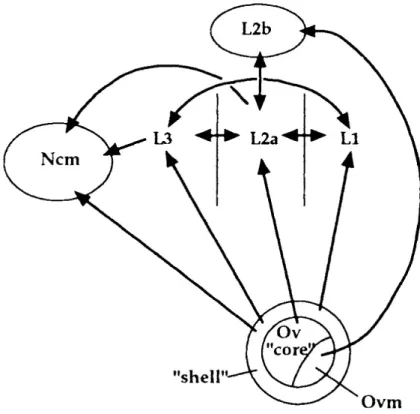
Broome, Mello, & Nottebohm, 1996). According to the research by Fortune and Margoliash in 1992, Field L can be splited into 4 cytoarchitectonically defined subdivisions: L1, L2a/L2b, and L3. The majority of Ov projections end in L2a and L2b (Adret et al., 2012). However, Ov is a bit more complex structure itself, and it is known to consist of ‘Ov core’, ‘Ovm’ and ‘Ov shell’ in more detail. Researchers found out that Ov core projects L2a, Ovm projects to L2b, and Ov shell projects to caudal medial nidopallium (NCM), L1 and L3. NCM and the field L subdivisions are most often connected in corresponding ways, as described in Figure 1.5. NCM has corresponding connection with Caudal mesopallium (CM) projecting to HVC and Nif. By far, this is known as a complete representation of all connections possibly delivering auditory information. Here we do realize that auditory information is distributed expansively over a large area of songbird telencephalon.
Indeed, auditory stimulation induces neural responses in the areas of the auditory pathway as well as the areas of the anterior forebrain pathway (A. J. Doupe &
Konishi, 1991; Allison J. Doupe, 1997) and in premotor areas (Katz & Gurney, 1981; McCasland & Konishi, 1981).
Figure 1.5.: Auditory input to the telencephalon regions in songbirds.
The caudal medial nidopallium and Field L are targeted by
auditory information originated from the thalamic nucleus Ov
“core” (Ovoidalis). Figure taken from (Vates et al., 1996).
In higher order area such Nif, the neural response for the birds own song (BOS) shows surprisingly higher preference over any other auditory stimulus and it is still unexplained phenomenon ((Bauer et al., 2008). On the other hand, neurons in CM and Field L were reported to convey motor related signals during singing. For example, they fired according to an anticipated vocal output or even to the contrast between the predicted and the actual output (G. B. Keller
& Hahnloser, 2009). Overall, the gathered data stresses the close interconnection of motor control and auditory processing in the songbird telecephalon.
Areas related to the motor pathway (MP) are essential for song production. In adult songbirds, lesion in any of the regions along the MP results in alteration or even loss of song output. Production of song moreover is required to be convoyed by premotor neuron’s activity time-locked to song. With no doubt, a direct neural connection must exit between the muscles of the syrinx and an area of the motor pathway.
Nottebohm et al. showed that hypoglossal neurons of nXIIts nucleus innervate to syringeal muscles (Nottebohm, Stokes, & Leonard, 1976). They further tracked neuron projections from nXIIts to the correspondent to primary motor cortex in mammals, the robust nucleus of the arcopallium (RA) in songbird. It is known that RA innervation of nXIIts is topographic (Blair Simpson & Vicario, 1990). RA neurons fire a sequence of short and sparse bursts of APs time-locked to a song motif during singing ((Dave, Yu, & Margoliash, 1998).
Major input to RA is rendered by the lateral magnocellular nucleus of the anterior nidopallium (LMAN) and premotor area HVC (used as a proper name) (Nottebohm, Paton, & Kelley, 1982). Nottebohm et al. and Bottier et al. also revealed that only HVC and RA are compulsory for adult song production but not LMAN by lesioning RA and its afferent regions (Bottier, 1984) (Nottebohm et al., 1976). Much like in RA, Neural activity in HVC is highly associated with song structure but considerably sparser in HVCRA (RA projecting neurons from HVC) neurons than in RA neurons. Only one high frequency AP burst is fired by HVCRA
neurons per motif (Richard H R Hahnloser, Kozhevnikov, & Fee, 2002;
Kozhevnikov & Fee, 2007). Participation in the singing related regions afferent to HVC is more modulatory and less understood.
HVC has direct innervation from the interface nucleus of the nidopallium (Nif) (Nottebohm et al., 1982). While irreversible Nif pharmacological lesions do not significantly impair the production of songs (Cardin & Schmidt, 2004), it has lately been shown that reversible pharmacological inactivation leads to a reduction in song stereotypes in the brief term (Naie & Hahnloser, 2011). While the bird is singing, Nif neural activity increases (McCasland, 1987).
The uvaeformis (Uva) thalamic nucleus projects directly and indirectly (via Nif) to HVC using two separate neuron projection classes (Akutagawa & Konishi, 2010;
Nottebohm et al., 1982; C. Z. H. Wang, Herbst, Keller, & Hahnloser, 2008).
Williams and Vicaro reported that singing related Uva activity discovered in chronic multi-unit recordings and modified song structure followed by electrolytic Uva lesions (Williams H, 1993).
However, it is not fully understood about a critical participation in song motor control of subsidiary HVC-afferent areas or afferent to any other motor pathway areas.
The anterior forebrain pathway (AFP) is a basal ganglia (BG)-thalamocortical loop bridging the cortical LMAN, the dorsal lateral nucleus of the medial thalamus (DLM), and Area X, which is homologue to BG of the mammalian). An input signal is provided to AFP by HVCX (Area X projecting neurons from HVC) neurons (Nottebohm et al., 1976). Nottebohm et al also showed neurons from LMAN are projecting to the RA and sending AFP output (Nottebohm et al., 1982).
In consistency with BG-thalamocortical architecture in mammalian circuits that were known to be involved in motor learning (Middleton & Strick, 2000) (JB, 2006), the AFP plays a significant role in song learning. An early research disclosed that pre- or during song learning lesions of the entire magnocellular nucleus of the nidopallium (MAN) have serious impacts on song learning and production while lesioning MAN in adult birds leaves song development intact (Bottjer & Arnold, 1984). Subsequently, Nordeen and Nordeen report that slow song deterioration is led by deafening in adult birds (Nordeen & Nordeen, 1992).
However, they also showed that LMAN lesion can partially prevent the deterioration (Nordeen & Nordeen, 2010). By far, several studies indicate that AFP
is accountable for creating exploratory variability and is therefore required for song learning (Andalman & Fee, 2009; Kao, Doupe, & Brainard, 2005; Ölveczky, Andalman, & Fee, 2005).
A set of HVC neurons that projecting to Area X provide input from MP to the AFP.
Therefore, HVC is thought to play a key role, i.e. by conveying motor information to the AFP. By now, HVC has been most intensively studied area in songbird brain.
In the next section, we will take a closer look at HVC.
1.1.4. The Cortical Premotor Area HVC
As seen in Figure 1.4, HVC is superficially located on the posterior pallium. Hence, it has been an easy subject for songbird researchers and deeply studied in the past.
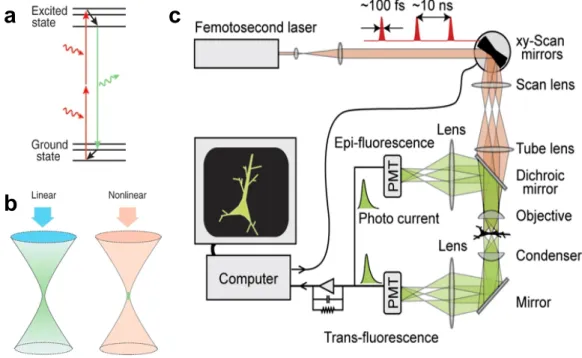
Even nowadays, most recent imaging researches still focus on HVC because it is the sole brain area involved in singing which can be reachable by optical methods until 2009 when the GRIN lens was highlighted as a deeper brain region imaging method (Barretto, Messerschmidt, & Schnitzer, 2009). However, the GRIN lens requires removal of tissues in the pathway from the brain surface to the target region. Therefore, HVC is the least affected brain area for optical imaging. This particular advantageous characteristic suggests accessing the functional microscopy techniques to deal with questions concerning HVC, including on motor control and on auditory processing.
In this section, we will take a closer look at HVC to set the foundation for the questions we want to examine utilizing in vivo single- and two-photon calcium imaging.
HVC connectivity
The anatomy of songbird's brain itself provides a clue about the significance of HVC for singing. First, HVC has a direct pathway to the syrinx muscles through nXIIts (see fig. 1.4). Second, HVC receives auditory inputs from various and diverse neural pathways. And lastly, HVC is originally a source of input to a basal ganglia-thalamocortical loop which also provides input to the motor pathway.
This is a rough description of HVCs connectivity. Here are some more in-depth fact about HVC: input from the medial magnocellular nucleus of the anterior nidopallium (MMAN) is received in HVC (Nottebohm et al., 1982), is reciprocally
linked with RA (Roberts et al., 2008) as well with a subsection of CM, termed nucleus Avalanche (Av) (Akutagawa & Konishi, 2010; Bauer et al., 2008;
Nottebohm et al., 1982).
The projections to regions efferent to HVC derive from diverse populations of HVC projection neurons. We describe neurons with an established projection target with subscript letters specifying the target. For example, HVC neurons projecting to Area X, we describe this as HVCX neurons. Recently discovered set of HVCAv
neurons is estimated to contain a few hundred neurons (Akutagawa & Konishi, 2010) and its element is not well known.
HVCRA and HVCX neurons, however, are relatively largely proportioned and well- studied. Another major set of neurons with no projections outside of HVC was introduced firstly through electrophysiology in slices (Dutar et al., 1998). They are so called HVC interneurons or HVCI neurons.
HVC physiology
Three essential discoveries initiated intensive research of the neural basis of song learning and of singing in songbirds: First was the necessity of the gain of auditory memory of kin song (Tamura, 1964). The second was the necessity of acoustic feedback during song production in juvenile period (Konishi, 1965). And third, it has been discovered that a particular set of interconnected forebrain areas are compulsory for song production (Nottebohm et al., 1976). Notably, early electrophysiological studies tried to discover areas of auditory processing and acoustic memories in songbird brains. Auditory responses to the introduction of noise and tone bursts in HVC neurons were first reported by Katz and Gurney (Katz
& Gurney, 1981), but they did not find which stimuli were favorable. Shortly after the first discovery, McCasland and Konishi found that even after the birds were deafened, their HVC showed signals of singing associated activities that were time- locked to a song. Additionally, McCasland and Konishi discovered that HVC neurons respond more favorably to the BOS compared to the BOS played in reverse (rBOS). They also showed that activity patterns in HVC have not altered by auditory stimuli (including BOS) played back when the bird was singing. It appeared that auditory input to HVC while the bird was singing to be prohibited, which allowed a pure premotor function to HVC (McCasland & Konishi, 1981).
Margoliash's additional research confirmed the general preference for the BOS of
HVC neurons over a broad range of stimuli: 1) temporally and spectrally altered BOS, and 2) conspecific songs (CON). Feedbacks of HVC neurons showed high responsiveness to temporal and spectral features of the BOS (Margoliash, 1983), and also to the temporal sequence of individual syllables (Margoliash & Fortune, 1992).
It seems the downstream structured muscles connecting from the RAs to the syringeal to be weakly myotropic (Blair Simpson & Vicario, 1990). There is no hard proof for a topographical link, neither efferent nor afferent, in the case of HVC (Foster, Mehta, & Bottjer, 1997). Neither did multi-unit feedbacks to acoustic stimuli unfold the topographic structuring of HVC activity. On the other hand, BOS stimulation seems to induce similar responses within the whole HVC (Sutter &
Margoliash, 1994).
Auditory responses of neurons in HVC are nevertheless of astounding nature:
single-unit responses to acoustic stimulation with the BOS can be caused by impressive temporal precision, showing a few bursts of APs, and precisely time- locked to the song by millisecond precision (Huetz, Del Negro, Lebas, Tarroux, &
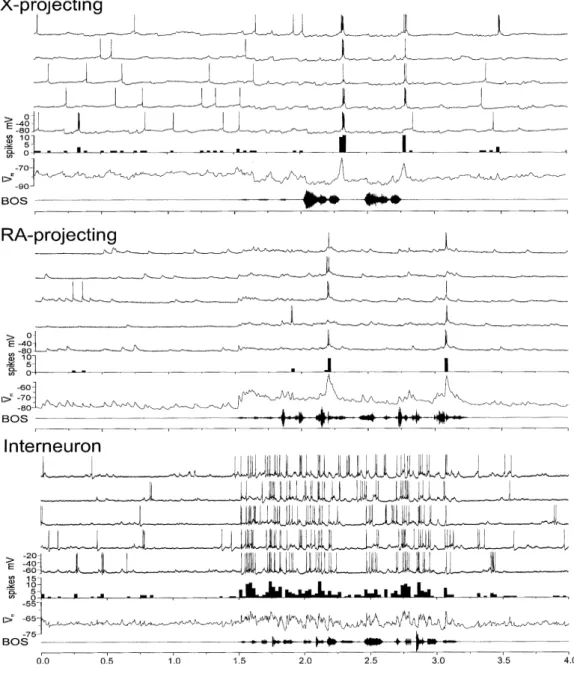
Edeline, 2006; Lewicki & Konishi, 1995; Mooney, 2000). Mooney explained the BOS responses of the three major types of HVCs neurons are different by showing the subthreshold mechanisms in anesthetized zebra finches. When HVCRA neurons are depolarizing during the entire song, HVCX neurons experience extended hyperpolarization. Even so, both neuron types express bursts that are time-locked within millisecond precision to the song playback (Mooney, 2000). HVCI neurons react with continuously increasing firing rate during the whole BOS stimulus.
Since anatomy provides different probabilities, speculation about the pathway on which acoustic information extend to HVC aroused. Irreversible excitotoxic lesioning (Cardin & Schmidt, 2004) of Nif and reversible inactivation (Coleman, Roy, Wild, & Mooney, 2007) steers to loss of acoustic feedback in HVC.
Electrophysiology showed that NifHVC neurons are particularly selective for the BOS similar to HVC neurons. Nonetheless, the sparse responses of HVC projection neurons to BOS arises solely within HVC. Nif responses are more retained and closely follow subthreshold HVC projection neuron responses (Coleman et al., 2007).
Figure 1.6.: Different types of HVC neuron responses selectively time- locked to BOS stimulation. Both HVCX and HVCRA projection neurons fire a small number of short bursts of AP, precisely time- locked to the song. However, while HVCRA neurons undergo strong depolarization during BOS playback, HVCX neuron responses exhibit sustained depolarization. On the other hand, HVCI neurons show increased firing rate during BOS playback. All neuron types are BOS specific. Figure adapted from (Mooney, 2000).
The HVC afferent thalamic region Uva has been revealed to gate BOS responses in HVC because electrical stimulation of Uva throughout BOS playback suppresses HVC BOS reactions, directly through HVC inhibition and indirectly through Nif input inhibition to HVC. Reversible Uva lesions do not critically influence HVC activity, neither voluntary nor in response to auditory input (Coleman et al., 2007).
Figure 1.7.: HVC activity in a bird that is singing and freely moving. On the top, there is a spectrogram of a motif of the bird's song. Below displays raster plots of various identified HVC neuron types. Horizontal lines break up individual neurons.
Every song variation can be seen on a new line. Throughout the song motif, HVCRA
neurons only fire on a strongly precise burst of APs, HVCX neurons display several brief bursts of APs, and HVCI neurons express more continuous but loosely song- locked firing. The figure above is taken from (Kozhevnikov & Fee, 2007).
CM is also involved in auditory input to HVC. Bauer et al. (Bauer et al., 2008) reported that auditory responses in HVC and Nif suppressed by CM inactivation.
Contrary to the above-mentioned studies, HVC auditory responses continued in this study even after irreversible Nif lesions. This indicates direct and immediate auditory input from CM to Nif.
To sum up, HVC auditory responses are affected by several interconnected afferent regions with direct projections to HVC. It seems there is no direct feedforward auditory pathway connected to HVC. Still, the remarkable characteristic of BOS selectivity of CM and Nif seems to emerge. CM is a bit BOS selective (Bauer et al., 2008) and Nif strongly BOS selective (Coleman et al., 2007). Nif is also highly selective for HVC projection neurons that respond with sparse and time-locked bursts of APs to a song. Therefore, there is a hierarchy in feature detection that culminates in the sparse depiction of sensory features in HVC, despite the lack of apparent hierarchical connectivity (Blättler & Hahnloser, 2011).
HVC premotor activity corresponds to the sparsity explained in the BOS stimulation responses and demonstrates the remarkable temporal precision of repeated song production in birds: Hahnloser et al. antidromically identified HVCRA neurons and recorded activity in freely behaving zebra finches. The downstream motor pathway is innervated by these neurons. Intriguingly, their premotor code is temporary; because each HVCRA neurons only fires a brief burst of APs once per each song motif (Richard H R Hahnloser et al., 2002; M. A. Long, Jin, & Fee, 2010), see Figure 1.7. Experiment results in which HVC has been cooled down further support the idea that HVC modulates the temporal precision firing to the bird's song. Cooling down HVC led in a song being dilated at all time scales (M. a Long & Fee, 2009), indicating a linear temporal code. This, however, opposes with the latest study suggesting that HVC could encode the production of vocal gestures (Amador, Stathopulos, Enomoto, & Ikura, 2013). Although this is an intriguing concept, this study depends heavily on the fit of a complicated physical model of song production and requires further research and support.
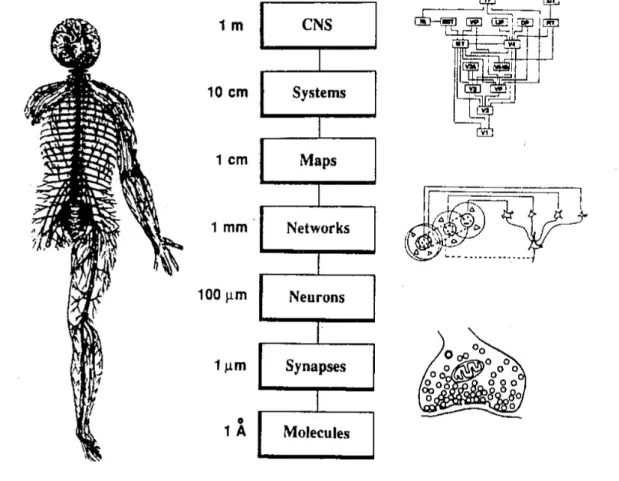
Figure 1.8.: Levels in spatial organization are schematically described in nervous systems. A whole nervous system of a sizeable vertebrate species is able to extend over several meters whereas the smallest functional elements are single molecules such as functioning ion channels. In between of them, we find systems, maps, networks, neurons, and synapses. Multi-photon microscopy empowers the study of neural phenomena in the extend of neurons to maps and now reaches to Synapses. Figure adapted from (Sejnowski, 1988).
When HVCRA neurons were selectively degenerated by neuron-type-specific method, the bird showed song deterioration and this confirms their important role as the primary HVC premotor output. On the contrary, singing is not impaired by the targeted ablation of HVCX neurons (Scharff, 2000). In addition, HVCX neurons also show sparse firing during singing, but several times per song motif unlike HVCRA (Kozhevnikov & Fee, 2007), see Figure 1.7. HVCx neurons are assumed to have a role in song learning since they project to the AFP. An intriguing research by Prather et al. (Prather, Peters, Nowicki, & Mooney, 2008) offers a little more light on their function: HVCRA neurons do not show responses to auditory stimuli
in freely behaving awake swamp sparrows, a songbird with a simple repertoire of song types; only HVCX neurons respond. Generally, HVCX neurons respond only to one version of the song repertoire, so called 'primary song'. The response to the song is precisely time-locked. These neurons simultaneously fire within the primary song while singing. Singing associated activity is unchanged by distorted auditory input and consequently 'corollary discharge'. Responses are also elicited by playing comparable songs from conspecific birds. This research defines HVCX
neurons as mirror neurons, much the same as mirror neurons in monkey's premotor cortex (Rizzolatti, Fadiga, Gallese, & Fogassi, 1996). Mirror neurons are considered important for learning imitations (Fabbri-Destro & Rizzolatti, 2008).
Idea that HVC is engaged in song learning suggests that structural modification occur during learning period within HVC. Roberts et al. observed that dendritic spines of spinous HVC neurons sustain and grow in size after the first exposure of a juvenile bird to the tutor's song1. In HVC projection neurons rapid spine turnover was identified in juvenile birds that were never introduced to a tutor song and ended about 60 days post-hatch, the age where the sensory stage is believed to end roughly in zebra finches, or after exposure to tutor song.
HVC neurons were virally infected with Green Fluorescent Protein (GFP) in the last-mentioned research and chronically imaged with a two-photon microscope. So far, a survey of neural activity in songbirds using two-photon microscopy has not been recorded. We present two-photon microscopy in the next chapter and how it allows neural activity research.
2. Song imitation performance in juvenile songbirds is uncorrelated with amount of practice
1 Both HVC projection neurons (HVCRA and HVCX) have spiny dendritic arborizations, whereas HVCI neurons are aspiny (Mooney, 2000).
2.1. Method
2.1.1. Experimental strategy
When juvenile zebra finches are at 15 days post hatch (dph), we separated adult males from the juveniles. This is because juvenile males typically enter a sensory song-learning period at this age (Immelmann, 1969). Then only their mothers in a soundproof recording chamber raised them until 41 dph for group 1 (n=17 birds) and 46-47 dph for group 2 (n=4 birds). This had the effect of isolating the juveniles from adult song (only male zebra finches sing). All birds (n=21 birds) did not undergo any surgery.
At approximately 42-47 dph, the song-isolate juveniles were exposed to a singing adult male tutor. Every morning for approximately 90 minutes, tutors were placed in the recording chamber in a separated cage adjacent to the juveniles. Each juvenile was alone in the chamber for the remainder of the day. This allowed us to acquire high-quality recordings when juveniles develop their songs. Therefore, juvenile males were exposed to only their tutors’ songs and their songs throughout the experiment. All vocal production inside the recording chamber were recorded and monitored.
2.1.2. Tutoring
For tutoring, we exposed a different adult male tutor to each juvenile. We minimized differences between the tutor songs by using only successfully tutored tutors by the same song playback in their cage where each song playback was triggered by a button. Juveniles were tutored with only live tutors instead of tutoring by song playback. This is because birds that learn from song playback alone showed low percentage of learning rate (see also Derégnaucourt et al., 2012).
In zebra finches, it is sufficient to make fairly complete imitation throughout giving a total daily duration of 30 seconds playback containing 40 tutor songs per day.
(Peters et al., 1992) (Tchernichovski et al., 1999). Typically, tutors in each tutoring session produced hundreds of song motifs in our experiment. This means that the 90 minutes exposure time to tutor was enough for achieving good imitation. Each juvenile was exposed to only one tutor across three weeks for group 1 and two weeks for group 2. Because song separation from multiple birds is not an easy task,
especially when the song of juvenile starts to imitate the song of tutor, we only analyzed vocal productions of juvenile in isolation without the tutor.
2.1.3. Song recordings
We recorded the vocalizations of juveniles throughout song development. Using customized Matlab base software, captured signal by a wall microphone was band- pass filtered between 100 Hz and 10 kHz. Then the filtered signal is digitized at a sampling rate of 32 kHz with 16-bit precision. In case of continuous recording by the software would result in 230 MB of data and it contains long recording time of non-song signals, redundant for our song analysis. Thus, we decided to record songs selectively and maximize the recording time of song only signals. We developed a method for identifying zebra finch vocalizations based on harmonic sound structure and separating them from other gratuitous sounds such as wing flaps. At every integer multiple of the fundamental frequency, harmonic sounds are characterized by signal intensities. The spectral sound density ɸ(ω) of the sound waveform was calculated as a function of sound frequency ω in 16-ms windows.
The harmonic power h is defined at a given frequency ω by the product of multiples and spectral densities at the frequency ω thereof:
ℎ(𝜔) = & ɸ(i ∗ ω).
!
"#$
We usually chose a total of N = 7 density multiples. Eventually, we defined the harmonic level as
𝐻 = 𝑚𝑎𝑥%ℎ(ω) 𝑚𝑖𝑛%!&'ℎ(ω()
which is higher for sounds with a harmonic structure than for broadband sounds of the same overall intensity. 𝐻(𝑡) was calculated as a function of the window center t discretized in 4 ms steps.
Whenever 𝐻(𝑡) was above a given threshold value during more than 50% of a 0.6 s time period, a save event was triggered. Then the recorded sounds were streamed to a file on a hard disk. Each recorded file started one second before the trigger event and ended after no trigger event was seen for an entire second (the criterion for song recording was evaluated every 4 ms). On a typical day we obtain about two hours of song recording data separated into 1500 files. Recorded files typically
contained bouts of vocalizations but individual calls or noises such as wing flaps were rarely found. Cage noise was unusually recorded only when intermingled with vocalizations. Our method based on the harmonic level performed superior song selection than with other methods based on sound amplitude alone (i.e.
threshold triggered song selection of the root-mean-square (RMS) of the sound waveform in 16 ms windows).
We inspected the histograms of harmonic detector levels for each day of recording (in days 50 – 60 post hatch) for all birds. Based on a visual inspection of the histograms, we estimate that ~5% of all vocalizations recorded during a day were rejected by our choice of threshold (50% above in a 0.6 s period).
2.1.4. Song Density
The amount of singing was computed by summing the durations of all song production from juvenile male in each day. A song is defined as a series of at least 3 consecutive syllables and automatically detected (each syllable is detected when it is at least 10 ms long with a gap of at most 500 ms to other syllables in juveniles).
Including 32 ms short margins added before the first and after the last syllable, the total song duration was defined as the interval exceeding 800 ms from the onset of the first to the offset of the last syllable. Superthreshold intervals of sound amplitude is defined as a root mean square sound waveform filtered between 500 Hz and 4 kHz and syllable is detected where the sound amplitude above the given threshold intervals.
2.1.5. Quantification of song development
To quantitatively measure and compare song development for each bird with tutor, we used a freely distributed Matlab based software package called Sound Analysis Pro (SAP). This software provides a tool for calculating the similarity between the tutor song and juvenile’s song (Tchernichovski et al., 2000), and has been implemented in many song analysis process and widely utilized in the songbird community (Benichov et al., 2016; Okubo, Mackevicius, Payne, Lynch, & Fee, 2015b; Pearre, Perkins, Markowitz, & Gardner, 2017). To estimate the similarity proportion of sounds in two different vocalizations, corresponded features such as frequency modulation, pitch, Wiener entropy, amplitude modulation, syllable duration, and spectral continuity are taken into account. In our analysis, we used the default parameter settings provided with SAP.
On the level of song motifs of the juvenile’s song and tutor song, song similarity values are computed with SAP. As SAP strongly depends on relative duration of motifs (typically similar length of motifs results larger similarity values), we restricted our similarity analysis to relatively matched duration of the juvenile’s song and tutor song motifs. In the next section, we describe a selection method to extract the most typical and representative motifs from variable juvenile songs.
To calculate song similarity on each analyzed day, we extracted the ten most representative (most typical) tutor song motifs. Using SAP, we calculated all 100 similarity values between the 10 x 10 pairings of tutor and juvenile motifs. The similarity values were small during the early plastic song phase and increased gradually from 50% at the beginning of tutoring to 80% on the last day of tutoring.
The standard deviation 𝜎 of song similarity values 𝑥" was calculated for the N=10 x 10 = 100 daily parings:
𝜎 = 5!$∑!"#$(𝑥" − 𝑥̅) eq.(1) where 𝑥̅ is a mean of the N similarity values. Also, the mean 𝜎9 and its standard deviation 𝜎) was calculated for n = 267 days (across 14/18 birds from experiment start until 60 dph):
𝜎9 = ∑*"#$𝜎" eq.(2-1)
𝜎) = 𝜎:1 − *,$+ ∙ =𝚪(n/2)
𝚪(𝑛−12 )>
+
eq.(2-2)
where 𝚪 is the gamma function.
For 4/18 birds, where we were not able to perform sufficiently reliable song selection because of large noise in the song recording, we manually inspected a random selection of 100 putative song motifs and selected the 10 best putative motifs for analysis.
We performed song similarity analyses starting about six days after tutoring onset, roughly when young birds produced rhythmic sequences of precursor syllables (Liu, Gardner, & Nottebohm, 2004; Okubo et al., 2015a). Note that our analysis avoided the sub-song phase (recordings before tutoring) during which we were
unable to select song motifs. This is because there is no validated method to perform song analysis at such a young age.
2.2.
Results
We exposed n = 18 juvenile male zebra finches each to the song of one adult male (n=6 tutors) zebra finch. At 15 days post hatch (dph), the juveniles were separated from adult male birds and placed in the care of their mothers. At around 30 dph, male juveniles from each clutch (family of juveniles + mother) were moved to individual acoustic isolation chambers. Starting at 45 dph (+/- 4 day), the pupils were exposed for 90 mins each day to adult male birds placed in a separate cage in their chamber, Fig 1A. Tutoring continued for three weeks in 14/18 birds and for two weeks in 4 birds due to logistical constraints. All vocalizations with a minimum amount of harmonic content (see Methods, Harmonics detection) were recorded and classified as either directed singing (the tutor was present), or undirected singing (the juvenile was alone). For practical reasons, juvenile birds were continually recorded for a variable number of days from a minimum of 64 days (2 birds) to a maximum of 95 days post hatch (2 birds).
We segmented any sound elements from background noises by thresholding sound amplitudes (root mean square of filtered microphone signal). We separated syllables from noise (wing flap etc.) using a semi-supervised clustering approach (see Methods). In 14/18 birds, we semi-automatically clustered song syllables into their distinct types by backtracking from the last day of recording because older birds produce more stereotyped song motifs, which allowed us to cluster song syllables, calls and introductory notes using a nearest neighbor method applied to spectrograms projected onto the top 20 principal components. After clustering a particular day (e.g. the last day), we used the clustered syllables to cluster the precedent day. Obtained clusters were manually corrected by visual identification of outliers. In 4/18 birds, we did not perform clustering because of excessive acoustic variability and recording artifacts. The total duration of putative singing on a given day was given by the summed duration of syllables (without silent gaps) within bouts.
a
b
c
d
Figure 2.1. Juveniles gradually improve the quality of their songs. (a) Birds were housed in acoustic isolation from 30 days post hatch (dph) on and exposed to their tutor for 90 minutes every day for three weeks starting (on average) from 45 dph. We longitudinally recorded their vocalizations. (b) Song similarity of candidate song motifs for the same bird as a function of time since experiment start. The black line marks the three weeks tutoring period. (c) The duration (in seconds) of putative singing produced by b13r16 as a function of time since experiment start. (d) Example log-power spectrograms of putative song motifs produced by the juvenile b13r16 with increasing duration of the experiment (top to bottom). For reference, two tutor motifs are shown on the top and the bottom. These were among the daily best candidates for the computation of song similarity, in terms of spectral and temporal stereotypy.
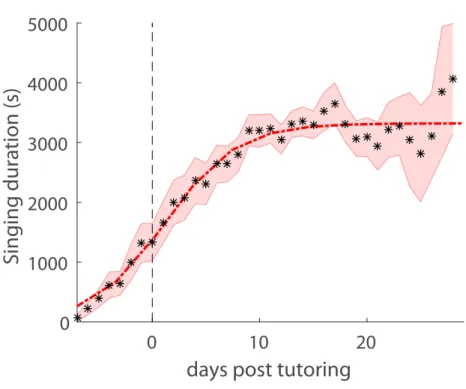
We found that song production increased rapidly after experiment onset and plateaued within two weeks at an average of 2.92 ± 0.56 x 103 s/day (average over 10-25 days since tutoring with data from a minimum of 7 birds), Fig 2a. The total tutor song produced by the tutor in the presence of the juvenile was 1.36 ± 8.23 x 103 s/day (n=4 tutors).
a
50 60 70 80 90
Pearson r : -0.046 p : 0.75 n = 18
a b
d
30 40 50 60 70 80
% similarity
1 1.5 2 2.5 3 3.5 4 4.5
singing duration(s)
104
1 2 3 4 5 6
Increment in duration (s)
×104 -20-10 0 10 20 30 40 50
Increment in Similarity (% )
50-60 dph 60-70 dph 70-80 dph 80-90 dph
n = 18 n = 18
n = 15
n = 9 n = 9
days post hatch (dph)
0 1 2 3 4
total tutor song (s)
×104 -100 10 20 30 40 50
Similarity from 50 to 60 dph (%)
Pearson r: -0.13 p : 0.6 , n = 18
0 10 20
days post tutoring
01000 2000 3000 4000 5000
Singing duration (s)
c
b
c
50 60 70 80 90
Pearson r : -0.046 p : 0.75 n = 18
a b
d
30 40 50 60 70 80
% similarity
1 1.5 2 2.5 3 3.5 4 4.5
singing duration(s)
104
1 2 3 4 5 6
Increment in duration (s) ×104 -20
-10 0 10 20 30 40 50
Increment in Similarity (%)
50-60 dph 60-70 dph 70-80 dph 80-90 dph
n = 18 n = 18
n = 15
n = 9 n = 9
days post hatch (dph)
0 1 2 3 4
total tutor song (s)
×104 -100 10 20 30 40 50
Similarity from 50 to 60 dph (%)
Pearson r: -0.13 p : 0.6 , n = 18
0 10 20
days post tutoring
01000 2000 3000 4000 5000
Singing duration (s)
c
50 60 70 80 90Pearson r : -0.046 p : 0.75 n = 18
a b
d
30 40 50 60 70 80
% similarity
1 1.5 2 2.5 3 3.5 4 4.5
singing duration(s)
104
1 2 3 4 5 6
Increment in duration (s) ×104 -20
-10 0 10 20 30 40 50
Increment in Similarity (%)
50-60 dph 60-70 dph 70-80 dph 80-90 dph
n = 18 n = 18
n = 15
n = 9 n = 9
days post hatch (dph)
0 1 2 3 4
total tutor song (s)
×104 -100 10 20 30 40 50
Similarity from 50 to 60 dph (%)
Pearson r: -0.13 p : 0.6 , n = 18
0 10 20
days post tutoring
01000 2000 3000 4000 5000
Singing duration (s)
c
d
e
0 1000 2000 3000 4000 5000 6000
20 10 0 10 20 30 40 50
prev day song duration (s)
Change in similarity
n=15 birds r: 0.045 p: 0.464
50 60 70 80 90
Pearson r : -0.046 p : 0.75 n = 18
a b
d
30 40 50 60 70 80
% similarity
1 1.5 2 2.5 3 3.5 4 4.5
singing duration(s)
104
1 2 3 4 5 6
Increment in duration (s) ×104 -20
-10 0 10 20 30 40 50
Increment in Similarity (%)
50-60 dph 60-70 dph 70-80 dph 80-90 dph
n = 18 n = 18
n = 15
n = 9 n = 9
days post hatch (dph)
0 1 2 3 4
total tutor song (s) ×104 -10
0 10 20 30 40 50
Similarity from 50 to 60 dph (%)
Pearson r: -0.13 p : 0.6 , n = 18
0 10 20
days post tutoring 0
1000 2000 3000 4000 5000
Singing duration (s)