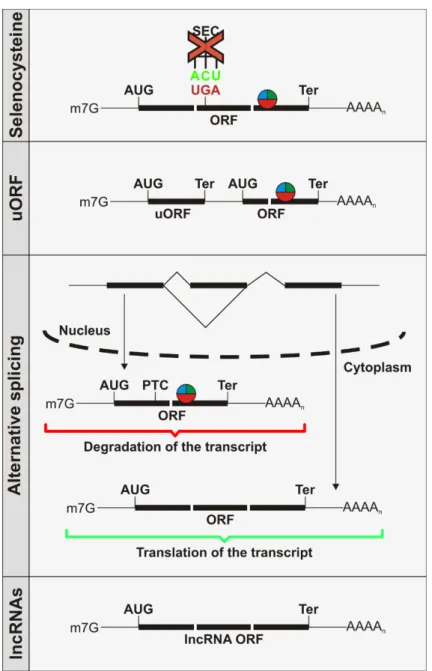
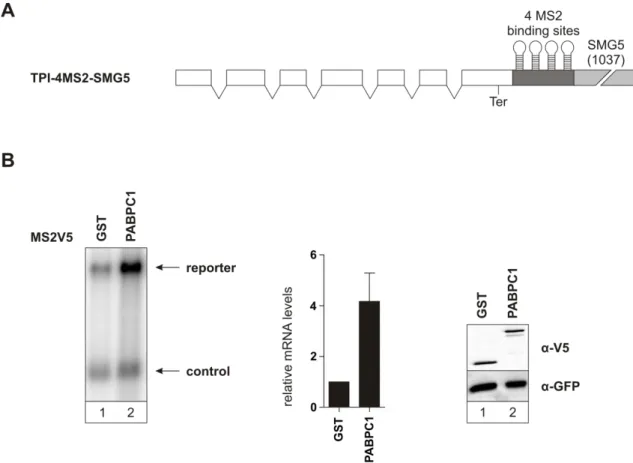
Inhibition of nonsense-mediated mRNA decay by the cytoplasmic poly(A)-binding protein
Volltext
Abbildung

ÄHNLICHE DOKUMENTE
We want to discuss the origin of the BRST symmetry in a more general context, and show that, by quoting Zinn-Justin, the ”Slavnov Taylor identities in gauge theories owe less to
3 Im Jahr 2010 werden die Leute nicht mehr rauchen dürfen.. In 2010 people will not be allowed to
The effect of indomethacin treatment on prema- ture labor was evaluated by cessation of uterine contractions and the period of prolongation of gestation.. Thirty minutes
This exercise sheet aims to assess your progress and to explicitly work out more details of some of the results proposed in the previous lectures. Please, hand in your solutions
Sehen Sie einen Zusammenhang mit der
Fachbereich Mathematik und
Adjoint functors between module categories are described by a tensor and a Hom functor and the properties derived from the categorical setting are explained in Section 3.. Algebras
der Universit at M unchen Set