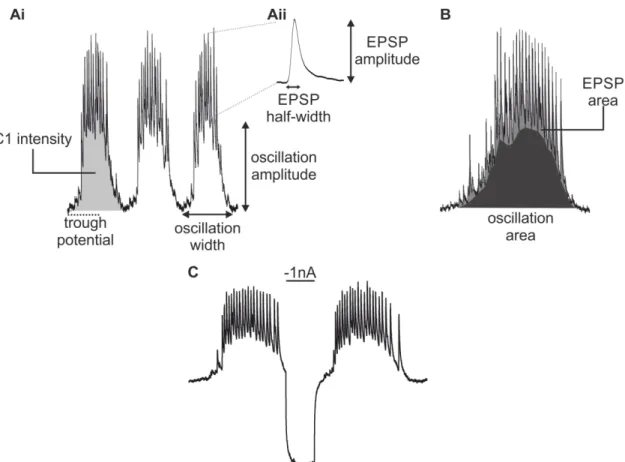
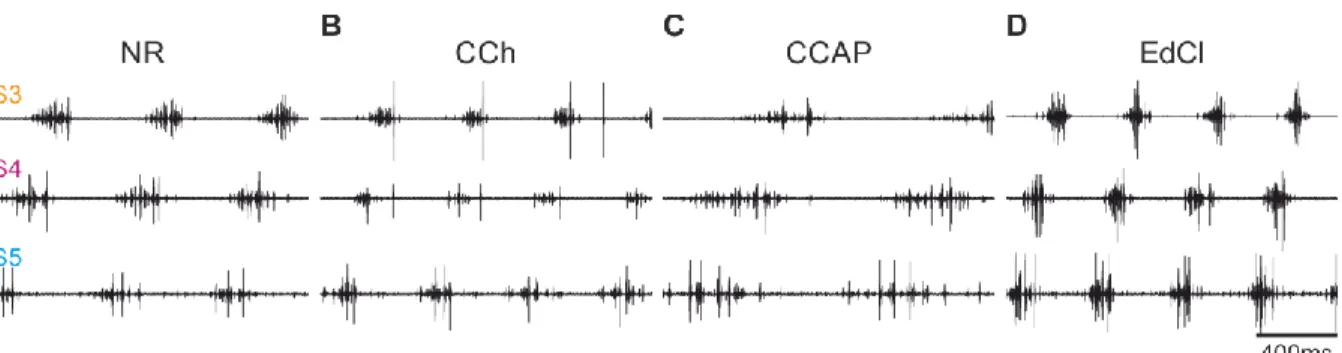
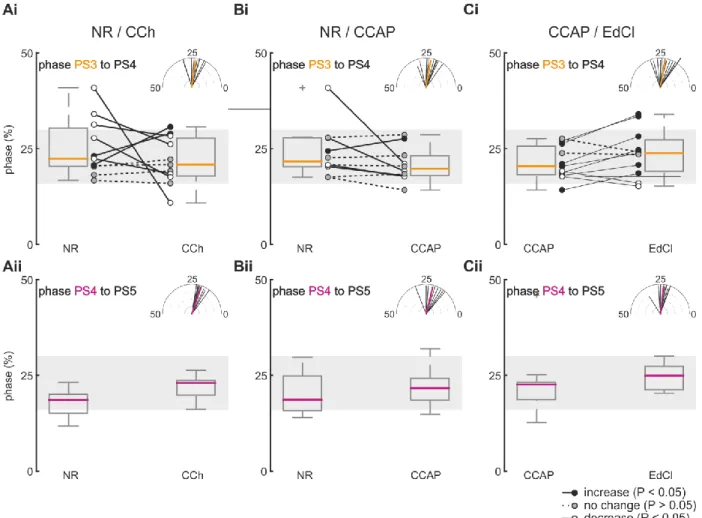
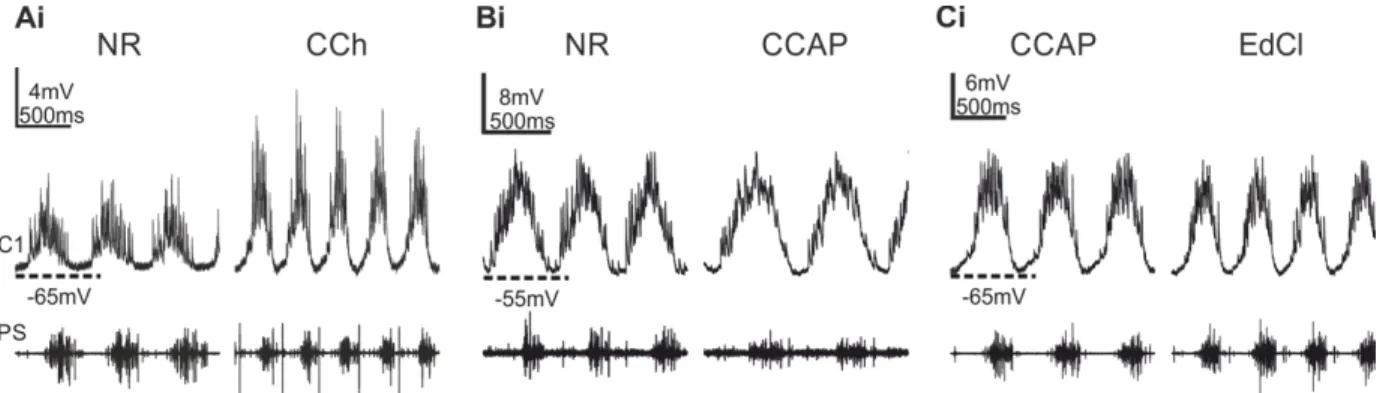
Physiological and morphological analysis of a coordinating circuit
Volltext
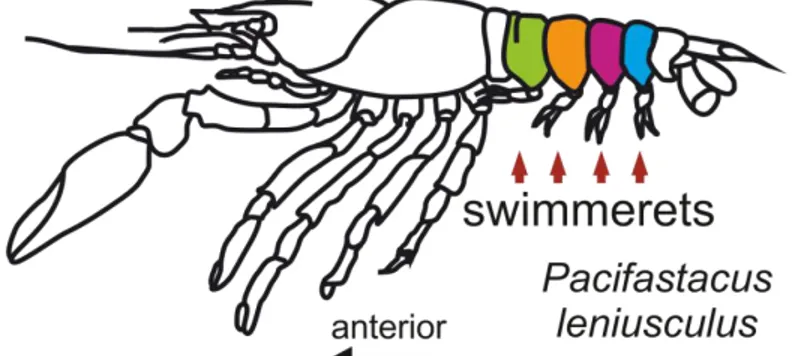
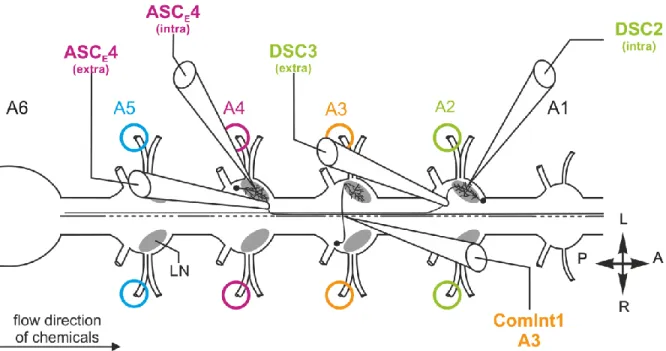
Abbildung


ÄHNLICHE DOKUMENTE
fimbriatus by its larger size (snout-vent length up to 200 mm vs. 295 mm), hemipenis morphology, colouration of iris, head and back, and strong genetic differentiation (4.8 %
ACL: Anterior cruciate ligament; ATT : Anterior tibial translation; EMG: Elec- tromyography; PTS: Posterior tibial slope; rTR: Range of tibial rotation; SLHD:.. Single-leg hop
In the calcium imaging, different glomeruli gave best responses to different odours, and each glomerulus corresponded in its response profile to one spike shape in the
Hattori S, Galushko AS, Kamagata Y, Schink B: Operation of the CO dehydrogenase/acetyl coenzyme A pathway in both acetate oxidation and acetate formation by the
1680s; Louis XIV; William of Orange; Count Imre Thököly; Ottoman Empire; Nine Years’ War; Glo- rious
Ultimately, a global response to the challenges posed by the international arms trade requires flexibility, creativity and innovation to develop national, regional, and
The study covers the following stages of dispute settlement: consultations, the request for the composition of a panel and the selection of panellists, the first written submissions
One of them, the European Banking Authority (EBA), is now empowered, inter alia, to perform a mandatory, legally binding mediation role to solve disputes between