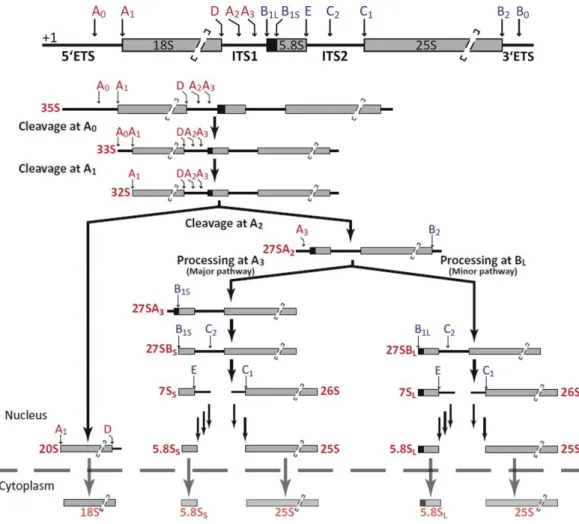
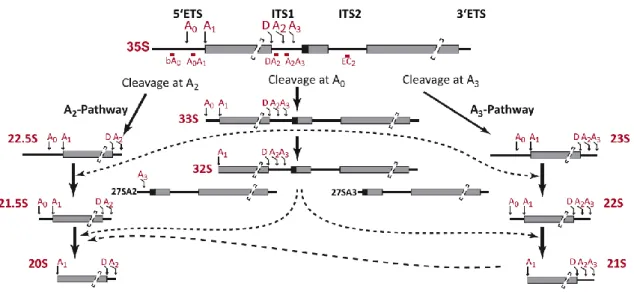
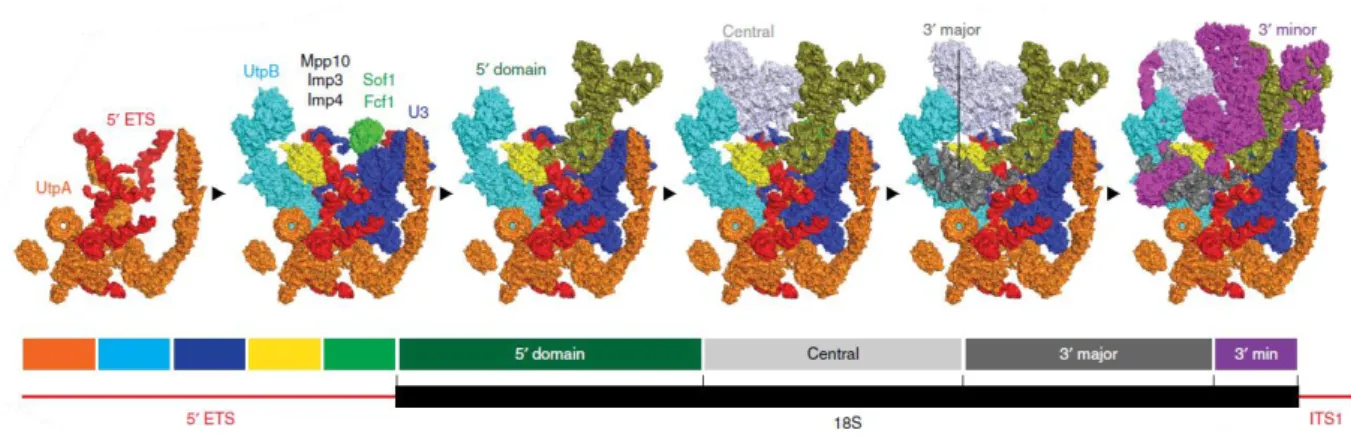
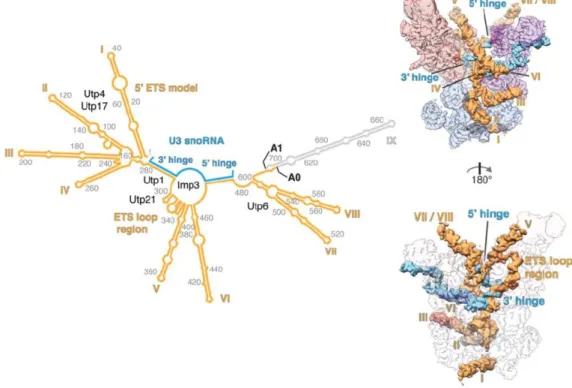
Functional characterization of Pol5 in the maturation of both ribosomal subunits
Volltext
Abbildung


ÄHNLICHE DOKUMENTE
"Community Medicine" aufgebaut. Ein Eckpfeiler dieses Schwerpunktes ist die Integration der Problemstellungen der Lehre, Forschung und medizinischen Versorgung.
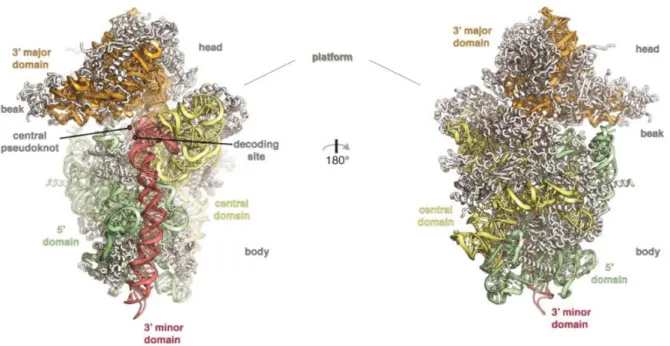
Based on these data and on structures of isolated L12, it was envisioned that the stalk is organized into three structural and functional elements, that are connected by
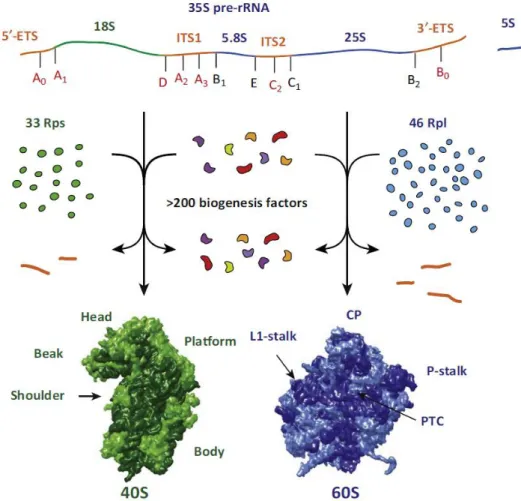
YbeY is a putative ribosomal endoribonuclease which has been implicated, among other things, to be involved in quality control of 70S ribosomes, in 17S pre-rRNA maturation
Dynamic instability results from GTP hydrolysis within the beta tubulin subunit that occurs upon assembly and destabilizes the lattice by
However, one has to keep in mind that Creld mutant flies have a higher mitochondrial mass, which is reflected in a higher ratio of protein content of the mitochondrial fraction
Additionally, pbl27 plants were also impaired in resistance against the bacterial pathogen Pst DC3000 hrcC suggesting that PBL27 plays a role in signal transduction of a
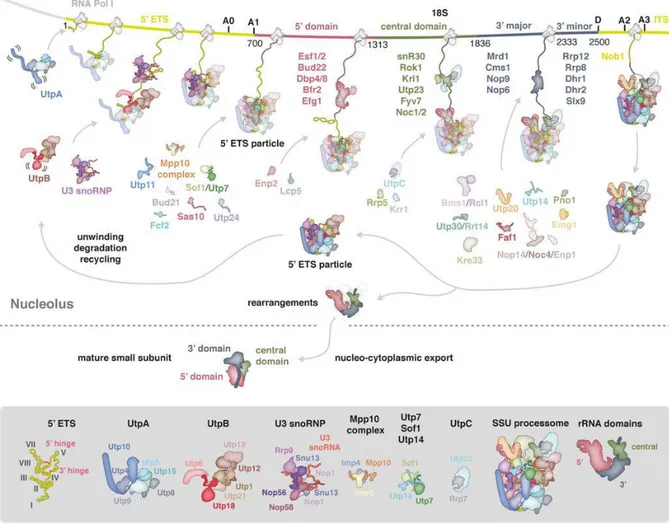
The observed CRAC sites for Has1 were consistent with its previously reported functions in the release the U14 snoRNA from pre-40S particles and regulating the release of a
To verify the indel within uL5 and its processed pseudogene, 1323 (uL5) and 346 (pseudo- gene) random cattle samples were genotyped using FRET 8 (primer sequences are listed in