Research Collection
Doctoral Thesis
Nitriding and Re-oxidation Behavior of Zircaloy-4 at High Temperatures
Author(s):
Park, Sanggil Publication Date:
2020-12
Permanent Link:
https://doi.org/10.3929/ethz-b-000459694
Rights / License:
In Copyright - Non-Commercial Use Permitted
This page was generated automatically upon download from the ETH Zurich Research Collection. For more information please consult the Terms of use.
ETH Library
Diss.ETH No. 27146
Nitriding and Re-oxidation Behavior of Zircaloy-4 at High Temperatures
A thesis submitted to attain the degree of DOCTOR OF SCIENCES of ETH ZURICH
(Dr. sc. ETH Zurich)
presented by Sanggil Park
MSc in Nuclear Engineering, ETH Zurich – EPF Lausanne born on 03.06.1986
citizen of Seoul, Republic of Korea
accepted on the recommendation of
Prof. Dr. Horst-Michael Prasser (ETH Zurich), examiner
Prof. Dr. Hans Jürgen Seifert (Karlsruhe Institute of Technology), co-examiner Dr. Jonathan Birchley (Paul Scherrer Institute, formerly), co-examiner
2020
Abstract
Zirconium alloys offer excellent properties for use as fuel cladding tubes and other structural materials in nuclear reactors. But, it has long been recognized that, in accident scenarios, overheated cladding undergoes exothermic oxidation in steam, leading to generation of hydrogen. However, exposure to air can lead to accelerated oxidation since the effect of nitrogen degrades the oxide layer which hence becomes less effective barrier, resulting in faster oxidation kinetics. The knowledge collected in the past studies has helped to identify two major behaviors of nitrogen during air oxidation. One is the nitriding which forms a micro porous layer, and the other is the re-oxidation which results in a macro cracked oxide layer due to the volume changes leading to faster oxidation kinetics. However, both reactions occur simultaneously and it is hard to understand their mechanisms separately and interrelated.
The present PhD study performs the series of separate effect tests under different boundary conditions by separating each reaction phase, pre-oxidation, nitriding, and re-oxidation, respectively. In addition, tests under identical conditions were repeated to assure the reproducibility of the behaviors. The series of tests were conducted in two temperature regimes. One is the breakaway temperature regime (< 1050°C) and the other is the non-breakaway temperature regime (> 1050°C). Moreover, the extensive post-test investigations are performed to study possible formation of ternary Zr-O-N phase during the nitriding. Based on findings from analyses of test data, the behavior of nitriding and re-oxidation was discussed and the open issues were identified. The present PhD work contributes to firstly prepare a foundation to achieve an in-depth understanding and secondly open the chances of interesting future research topics who are interested in the nitriding and re-oxidation.
Zusammenfassung
Zirkoniumlegierungen bieten hervorragende Eigenschaften für die Verwendung als Brennstoffhüllrohre und andere Strukturmaterialien in Kernreaktoren. Es ist jedoch seit langem bekannt, dass in Unfallszenarien überhitzte Hüllrohre in Dampf exotherm oxidieren, was zur Erzeugung von Wasserstoff führt. Die Einwirkung von Luft kann dabei zu einer beschleunigten Oxidation führen, da die Einwirkung von Stickstoff die Oxidschicht abbaut, wodurch die Barriere weniger wirksam wird, was wiederrum zu einer schnelleren Oxidationskinetik führt. Das in früheren Studien gesammelte Wissen hat dazu beigetragen, zwei Hauptverhalten von Stickstoff während der Luftoxidation zu identifizieren. Eines ist das Nitrieren, das eine mikroporöse Schicht bildet, und das andere ist die Reoxidation, die aufgrund der Volumenänderungen, die zu einer schnelleren Oxidationskinetik führen, zu einer makrorissigen Oxidschicht führt. Beide Reaktionen treten jedoch gleichzeitig auf und es ist schwierig, ihre Mechanismen getrennt von- und miteinander zu verstehen.
Die vorliegende Doktorarbeit führt eine Reihe separater Effekttests unter verschiedenen Randbedingungen durch, indem jede Reaktionsphase, Voroxidation, Nitrierung bzw. Reoxidation getrennt voneinander wird. Zusätzlich wurden Tests unter identischen Bedingungen wiederholt, um die Reproduzierbarkeit des Verhaltens sicherzustellen. Die Versuchsreihen wurden in zwei Temperaturbereichen durchgeführt. Eines ist das Abreißtemperaturregime (< 1050°C) und das andere ist das Nichtabbruch-Temperaturregime (> 1050°C). Darüber hinaus werden umfangreiche Nachuntersuchungen durchgeführt, um die mögliche Bildung der ternären Zr-O-N Phase während des Nitrierens zu untersuchen. Basierend auf den Ergebnissen aus Analysen von Testdaten wurden die Verhaltensweisen von Nitrieren und Reoxidation diskutiert und die offenen Fragen identifiziert. Die vorliegende Doktorarbeit trägt dazu bei, zum einen eine Grundlage für ein tiefgreifendes Verständnis vorzubereiten und zum anderen die Chancen für spannende, zukünftige Forschungsthemen zu eröffnen, die sich mit dem Nitrieren und der Reoxidation befassen.
Acknowledgments
To God be the Glory
It has been really grateful time for me to everyone. If I address all of my gratitude, it will be never ending. I will briefly give my thanks to all who has sincerely supported me to run the race of PhD. For me, it has been also very difficult time to finish the thesis. When I departed Switzerland in 2016 with the first draft of thesis, I thought I will be awarded the PhD degree soon. After four years, however, now I can submit the thesis. Through the past four years, every night I has been in the fear and great stress about the thesis. Finally I hope to be free from this burden and to start my new life.
Terttaliisa: My forever boss. You always save my life. Through your leadership, I could start this work and thanks to your support, I could get married. My life is indebted to you.
Martin: My best teacher and my best friend. You are my best Martin. You are the great scholar and the kind friend for me and everyone. Thanks always.
Jon: Best scientist in the history, for sure in my life history. Your kind and tolerance and best guidance, it has opened my life to go forward. Thanks my teacher, Jon.
Bernd: Without your further effort on this project, it was impossible to improve the thesis and knowledge. I greatly desire to see your model and hope this thesis will be useful to you.
Leticia: I miss you, and I wish all the best your life. It was very grateful time for me to be supervised by you for this great work.
Prof. Seifert: When I departed Switzerland in Feb 2016, I always remember your encouragement. Thanks to your encouragement, now I finally finish the thesis.
Prof. Prasser: If someone asks me, who do you respect most? I will answer, Prof.
Prasser. Since 2011, Prof. Prasser has been my academic father and always I am very proud of being his student. I will be the best and do the best as your student.
Contents
Chapter 1 Preface
11.1 Introduction 2
1.1.1 Motivation 2
1.1.2 Past studies 3
1.1.2.1 Past studies for a nuclear application 3 1.1.2.2 Past studies for a fundamental knowledge of Zr-O-N system
and for more general application 9
1.2 Basic Knowledge 12
1.2.1 Metal oxidation reaction and its kinetics 12
1.2.2 Zr-O, Zr-N and Zr-O-N systems 17
1.2.2.1 Zirconium metal 18
1.2.2.2 Zirconium oxide phases 18
1.2.2.3 Zirconium nitride phases 19
1.2.2.4 Zirconium oxynitride phases 21
1.2.3 Zirconium oxidation and nitriding 26
1.3 Research questions 33
Chapter 2 Methods
352.1 Description of experimental setup 36
2.1.1 Setaram TAG system 36
2.1.2 Netzsch STA-409 system 38
2.1.3 Gases 40
2.1.4 Zircaloy-4 samples 40
2.2 Test procedure and matrices 42
2.2.1 Test procedure 42
2.2.2 Test matrices 44
2.2.3 Differences and limitations for both thermobalance tests 49 2.3 Critical evaluation of experimental methods 50 2.3.1 Quantities for the analysis of thermobalance tests data 50
2.3.2 Behavior of gas transport 52
2.3.3 Transition of reaction phase 62
2.4 Post-test examination methods and equipment 64
2.4.1 Metallography: optical microscopy 65
2.4.2 Raman spectroscopy 66
2.4.3 Scanning electron microscope and Energy dispersive x-ray spec
troscopy 68
2.4.4 Wavelength-dispersive spectrometers 69
2.4.5 X-ray photoelectron spectroscopy 69
2.4.6 X-ray power diffraction 69
Chapter 3 Results and Discussion
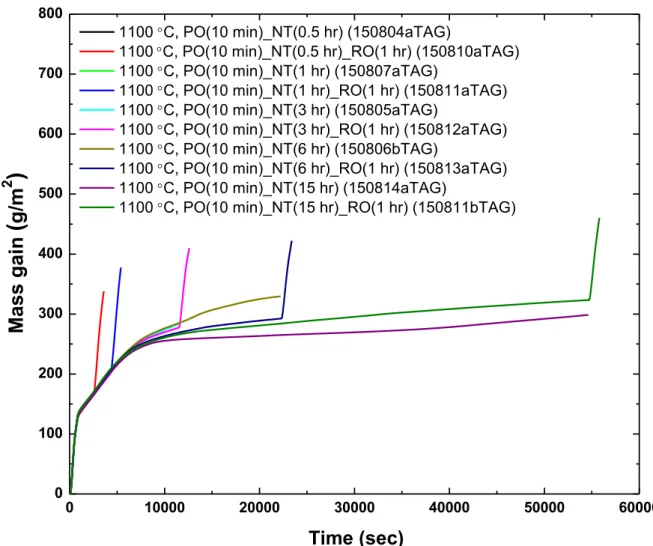
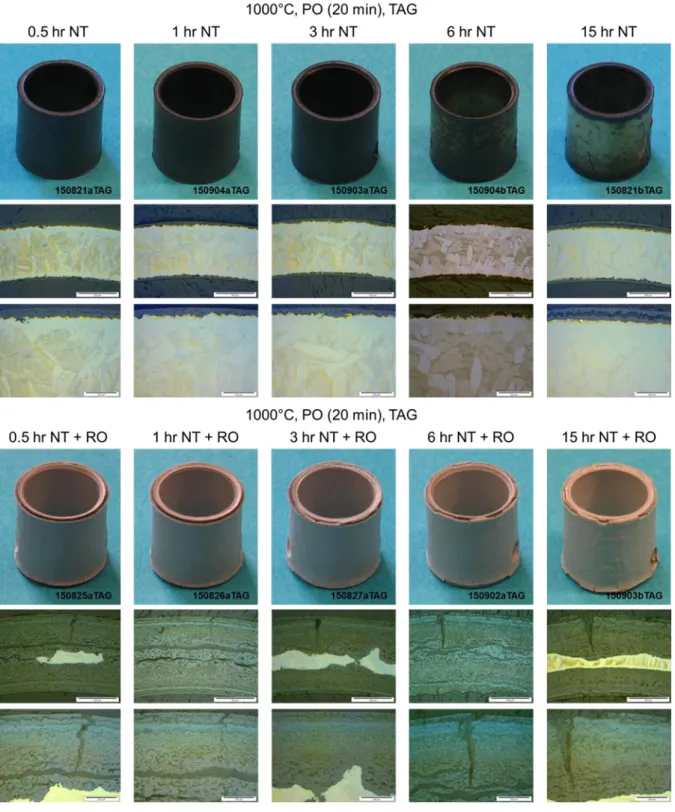
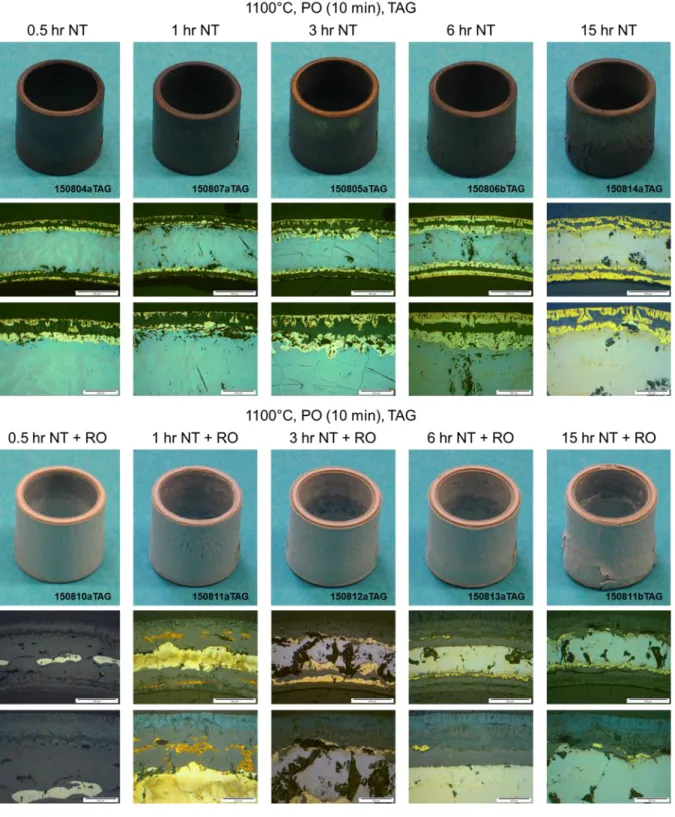
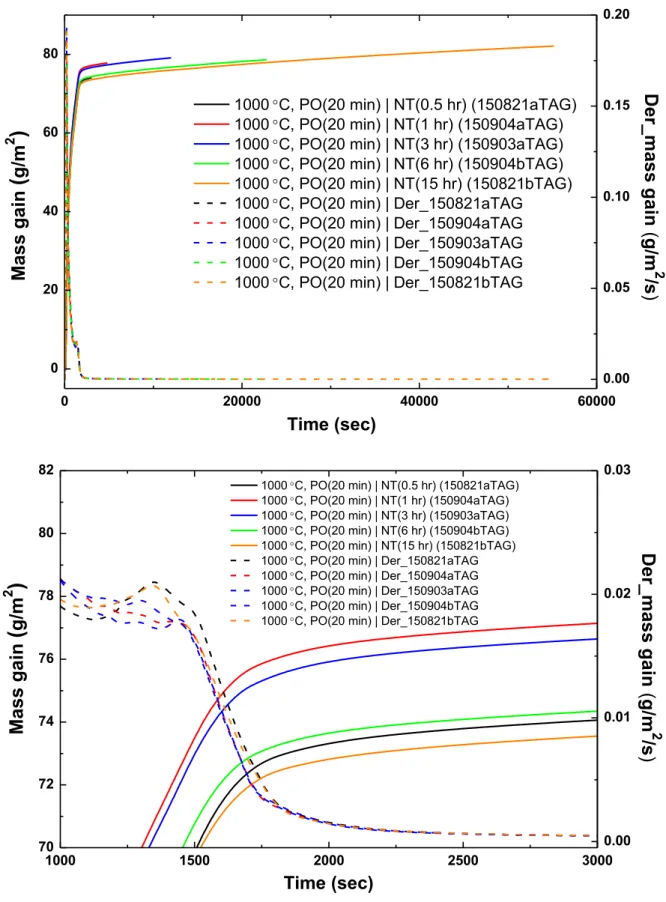
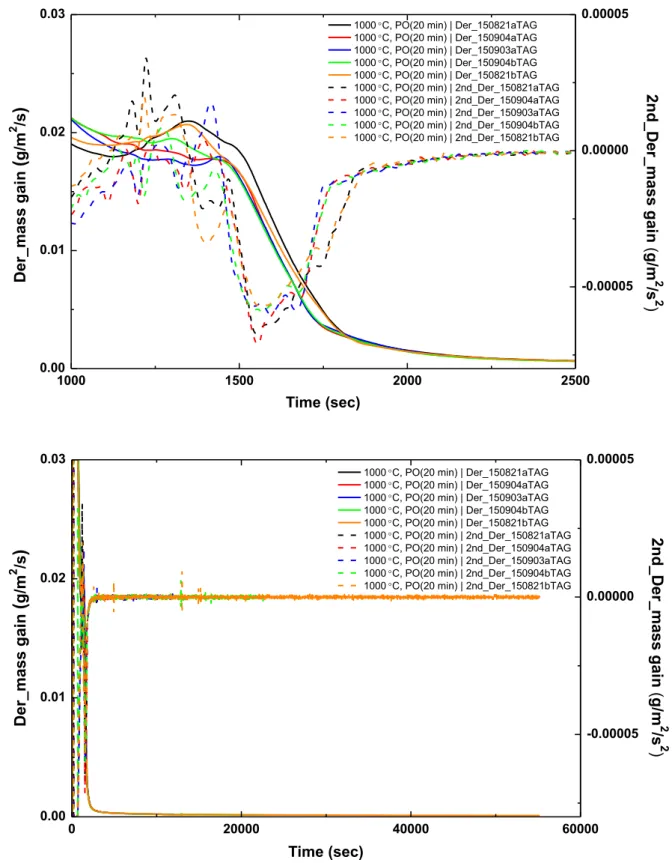
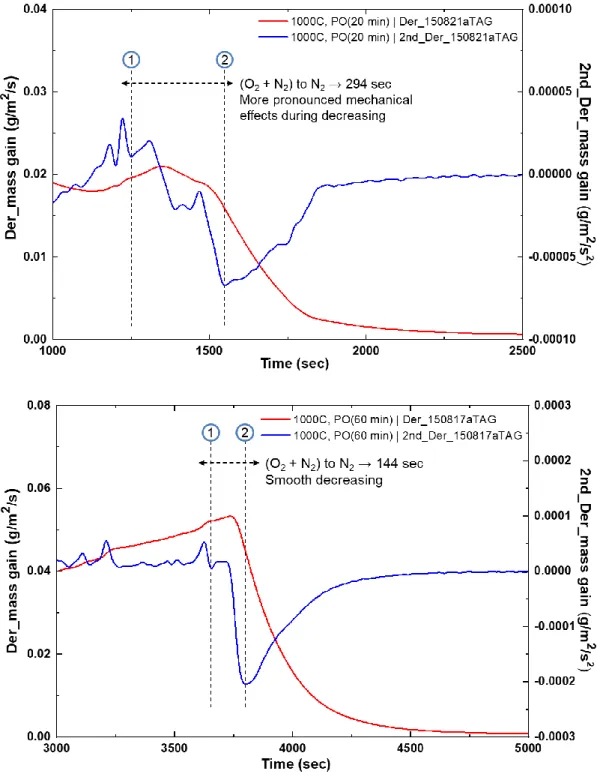
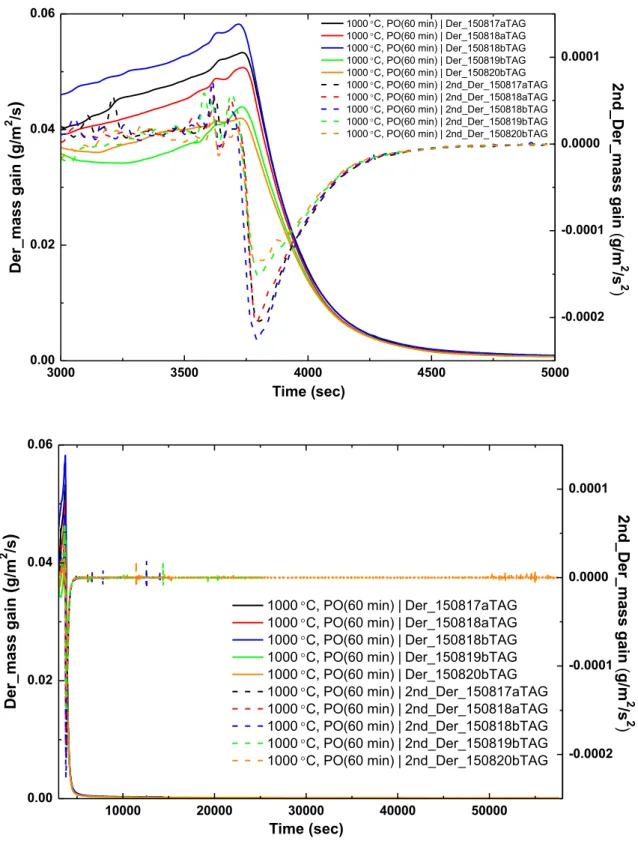
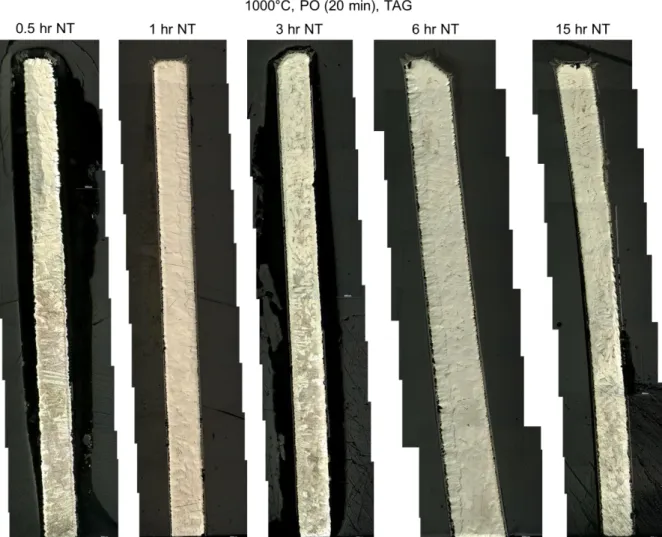
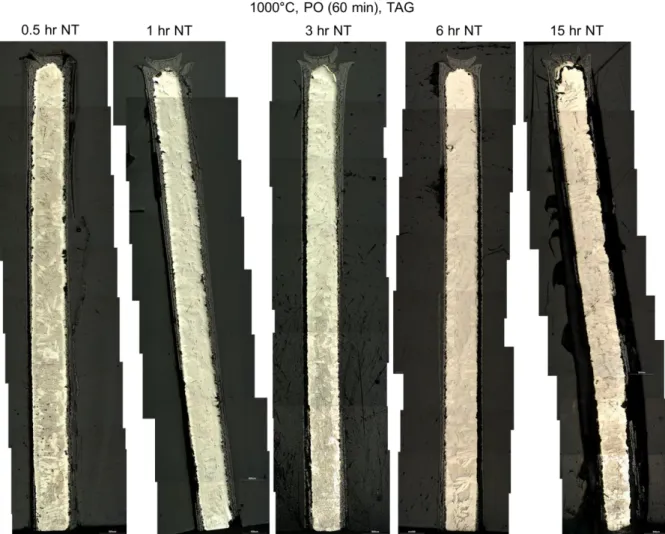
70 3.1 Analysis of test data from the Setaram TAG system 72 3.1.1 Analysis of the pre-oxidation phase in tests data from theSetaram TAG system 79
3.1.1.1 Pre-oxidation at 1000°C in the Setaram TAG system 80 3.1.1.2 Pre-oxidation at 1100°C in the Setaram TAG system 84 3.1.2 Analysis of the nitriding phase in tests data from the Setaram
TAG system 85
3.1.2.1 Nitriding at 1000°C in the Setaram TAG system 86 3.1.2.2 Nitriding at 1100°C in the Setaram TAG system 105 3.1.3 Analysis of the re-oxidation phase in tests data from the
Setaram TAG system 132
3.1.3.1 Re-oxidation at 1000°C in the Setaram TAG system 135 3.1.3.2 Re-oxidation at 1100°C in the Setaram TAG system 151 3.2 Analysis of test data from the Netzsch STA-409 system 168
3.2.1 Analysis of tests data at 900°C 168
3.2.2 Analysis of tests data at 1000°C 171
3.2.3 Analysis of tests data at 1100°C 175
3.2.4 Analysis of tests data at 1200°C 179
3.3 Post-test investigation: phase and elemental analyses 188
3.3.1 Phase analysis by Raman investigation 189
3.3.1.1 Reference sample Raman spectra 189
3.3.1.2 Raman data analysis methodology 191
3.3.1.3 Raman data analysis 192
3.3.2 Elemental analysis by various methods 210
3.3.2.1 Energy-dispersive X-ray spectroscopy (EDS) 210 3.3.2.2 Wavelength-dispersive X-ray spectroscopy (WDS) 211
3.3.2.3 Scanning electron microscopy (SEM) 214
3.3.2.4 X-ray photoelectron spectroscopy (XPS) 216
3.3.2.5 X-ray powder diffraction (XRD) 222
Chapter 4 Mechanistic understanding of behavior and
open issues
2254.1 Mechanistic understanding of behavior and open issues 225
4.1.1 Pre-oxidation 226
4.1.2 Nitriding 229
4.1.3 Re-oxidation 239
4.2 Open issues and future study 245
4.2.1 Quantification of the already developed alpha-Zr(O) during PO
phase 245
4.2.2 Quantification of the further developed alpha-Zr(O) during NT
phase 246
4.2.3 Sample’s edge effect on the reaction behavior 252 4.2.4 Resolution of the amount of NT phase mass gain that was uptake
of residual oxygen 254
4.2.5 Experimental conditions as compared to a reactor and/or spent fuel
pool case 254
Chapter 5 Conclusion and Outlook
256Appendix I. Mass balance analysis of test data 260
References 267
1
Chapter 1 Preface
The present thesis aims to investigate the behavior of nitriding and re-oxidation of zircaloy-4 at high temperature. This thesis contains mostly the experimental results of thermobalance tests and the post-test sample analyses to understand in more detail the phenomena during nitriding and re-oxidation.
The thesis consists of five chapters: Chapter 1, Preface; Chapter 2, Methods; Chapter 3, Results and Discussion; Chapter 4, Mechanistic understanding of behavior and open issues; Chapter 5, Conclusion and Outlook.
Chapter 1 provides the background of the present PhD study and the related past studies to present the current status of knowledge and research questions to be investigated in this thesis. Chapter 2 explains the experimental method to solve the research questions raised in chapter 1. Chapter 3 presents the experimental results and discussion to understand better the phenomena of oxidation and nitriding. Chapter 4 presents a mechanistic understanding of behavior by summarizing main findings from the experimental results and also presents the open issues which need further study in the future. Finally, chapter 5 provides conclusion of the present PhD study and its outlook how to use the findings and understandings of behavior of nitriding and re-oxidation from this thesis.
In this chapter 1, three sub chapters are provided. Chapter 1.1 introduces the motivation of present thesis and past studies of zirconium oxidation and nitriding researches. Chapter 1.2 summarizes the basic phenomenology of metal oxidation and provides a brief explanation of behavior of zirconium oxidation and nitriding. Lastly, chapter 1.3 identifies the research questions in the present PhD study.
2
1.1 Introduction
In this chapter 1.1, firstly motivation of the present thesis is introduced and then the trend of past studies of zirconium oxidation and nitriding are summarized.
1.1.1 Motivation
Zirconium alloys are used as cladding materials in light water reactors and acting as a safety barrier against a release of radioactive materials in case of reactor core uncovery in a severe accident scenario. It has long been recognized that overheated cladding undergoes exothermic oxidation in steam, leading to the generation of hydrogen. Under such circumstances, the oxidation rate would typically be limited by the rate of oxygen diffusion through the oxide layer which builds up on the cladding surface, provided the layer remains undamaged. However, exposure to air can lead to accelerated oxidation since the effect of nitrogen degrades the oxide layer which hence becomes a less effective barrier, resulting in faster oxidation kinetics.
There are situations where air ingress scenarios may occur: 1) during a severe accident the reactor pressure vessel could fail and air could ingress [1-2]; 2) during mid-loop operation when the reactor coolant system is usually opened to the containment [3] or 3) in spent fuel facilities such as pools and dry casks in either the event of loss of cooling or handling and transport accidents [4-6].
In order to study cladding degradation in the air ingress scenarios, numerous separate effect tests have been performed in the past to study the influence of nitrogen effect during oxidation. The knowledge collected in the past studies has helped to identify two major roles of nitrogen during air oxidation. The first role is the oxide layer degradation by forming a micro porous and macro cracked oxide due to the volume changes between the involved phases leading to faster oxidation kinetics. The second is the continued exothermic heat release by the nitriding in the absence of oxidant and the subsequent re-oxidation if the oxidant supply is restored (e.g. following water
3 injection).
The nitriding itself is an exothermic reaction and it yields an exothermically re- oxidizable nitride in the presence of newly coming oxygen. The nitriding is likely to occur under oxygen starvation condition where oxygen is sufficiently consumed. In addition, the nitrogen is readily incorporated into the oxidized metal rather than a bare metal. In the present thesis, the behaviors of nitriding and re-oxidation are experimentally investigated to understand better their mechanisms.
1.1.2 Past studies
In the past, the studies of zirconium oxidation and nitriding have been in various fields.
In this chapter, past studies are separately summarized for the nuclear application and for the fundamental knowledge of the Zr-O-N system, and for more general application, respectively.
1.1.2.1 Past studies for a nuclear application
The behaviors of zirconium and zirconium-based alloys in presence of steam has been investigated for a long time, and more recently, the reaction with oxygen and nitrogen has been studied for the safety issues raised in the air ingress scenarios.
Leistikow and Berg [7] conducted a Zircaloy-4 air oxidation tests at 750-1250°C. More than a decade later, experimental and analytical work on air ingress were performed in the frame of OPSA (Oxidation Phenomena in Severe Accidents) project [8] and COBE (Core Degradation Behavior) [9] in the late 1990s. In the OPSA project, the CODEX (Core Degradation Test) air ingress tests (AIT) were conducted with two experiments CODEX-AIT-1 (1998) and CODEX-AIT-2 (1999) using PWR 9-rod bundle [10-11]. In addition, CODEX-AIT-3 was recently conducted.
Later on, extensive separate effect tests (SETs) and Integral effect tests (IETs) have been performed in USA and Europe. Also, model developments for the reactor system
4
safety analysis codes (e.g. MELCOR, SCDAP, ASTEC, MAAP, ATHLET-CD and SOCRAT) were performed based on the tests data in several institutes and improvements are ongoing with further tests.
In USA, Natesan and Soppet (ANL; Argonne National Laboratory) [12-13] conducted air oxidation tests with Zr-based alloys in the temperature range of 300-600°C to represent the cladding heat-up in the event of partial or full draining of spent fuel pool.
Afterward, the Sandia Fuel Project was performed in the framework of an OECD/NEA project to simulate a spent fuel pool complete LOCA (Loss of Coolant Accident) with 17×17 PWR mock fuel assemblies. Tests consisted of Phase-1 with one assembly and Phase-2 with 1 heated and 4 unheated fuel assemblies to examine the propagation of fire [14].
At IRSN (Institut de Radioprotection et de Sûreté Nucléaire) in France, Duriez et al.
[15-17] launched the MOZART program to investigate the air oxidation of various type of Zr-based alloys with different initial states (as-received, pre-oxidized and pre- hydrided) at 600-1200°C mainly focusing on a status of spent fuel pool accident.
Coindreau et al. [18] modeled the air oxidation of Zircaloy-4 in the 600-1000°C temperature range into ASTEC code based on results obtained from the MOZART program. For better understanding the phase transformation in the air-oxidized samples, Idarraga et al. [19-20] investigated samples by Raman spectroscopy. More recently, Lassere et al. [21-22] has studied a degradation of Zircaloy-4 in air environment at 850°C and developed an air oxidation model considering the nucleation and growth for nitrogen-affected zones. In addition, the French DENOPI project has been launched in 2013 for 6 years duration to study a behavior of fuel assemblies in case of partial loss of coolant accident. In the DENOPI project, experiments have been performed in air/steam mixtures [23]. All research programs at IRSN mentioned here are aimed at developing better oxidation model in the ASTEC code.
At KIT (Karlsruhe Institute of Technology) in Germany, Steinbrück et al. have performed numerous SETs [24-30] and IETs [31-33] regarding the air ingress scenarios in both reactor and spent fuel pool sequences. SETs have been performed with various types of Zr-based alloys at temperature in the range of 800-1600°C to represent the in-reactor conditions in several oxidation environments (e.g. air,
5
air/steam mixture, oxygen/nitrogen mixture, nitrogen/steam mixture, air after steam and nitrogen after oxygen). Air ingress integral tests were conducted in the QUENCH program. The earlier test was QUENCH-10 with strongly pre-oxidized claddings. The QUENCH-10 test aimed to investigate the effect of air ingress on the heavily pre- oxidized claddings in steam, typical for spent fuel pool accident [31]. On the other hand, the QUENCH-16 test was conducted with limited pre-oxidized claddings. The QUENCH-16 test aimed to investigate the oxidation behaviors of Zircaloy-4 in air after rather moderate pre-oxidation in steam. The QUENCH-16 test included a long period of oxygen starvation to enable the nitrogen uptake [32-33]. In addition, the QUENCH- 18 test was performed to study the air ingress and aerosol release. Results obtained from tests at KIT have supported the air oxidation model developments in several institutes (e.g. PSI air oxidation model [34-36], an air oxidation model in ATHLET-CD code [37-38], in MAAP code [39-41] and in SOCRAT code [42-43]).
INR Pitesti in Romania conducted several SETs with the steam pre-oxidized Zircaloy- 4 in air, air/steam mixture, and steam environments in temperature of 900-1400°C [44].
In addition, IBRAE at Russia performed air ingress IET (PARAMETER-SF4 test) using E-110 cladding which is fuel type of VVER [44]. Table 1.1 and 1.2 summarizes main features of air oxidation tests introduced above.
Table 1.1 Main features of air oxidation tests (SET) Separate Effects Tests
ANL KIT IRSN INR
Zr-alloy Zry-4, Zirlo, M5
Zry-4, Zirlo,
M5 Zry-4, M5 Zry-4
Pre-oxidation Steam at 550°C
Steam or oxygen
or air at various temperature
Steam or oxygen
at 500°C
Steam at 600°C
Temperature
range 300-900°C 800-1600°C 600-1200°C 600-1400°C
6
Table1.2 Main features of air oxidation tests (IET) Integral Effects Tests
QUENCH
-10 QUENCH
-16 QUENCH
-18 SFP PARAMETER
SF4 CODEX
-AIT-1 CODEX -AIT-2
Zr-alloy Zry-4 Zry-4 Zry-4 Zry-4 E-110 Zry-4 Zry-4
Pre oxidation
Steam at 1620-1690K
for 113 min
Steam at 1300-1430K
for 4000 s
Steam at 1100-1400K
for 7540 s
None Steam at 1473K for 6886 s
Oxygen at 950°C for 100 s
Steam/air at 750 and
900°C for 3600 s
Air ingress
temp.
range
1190-2200K for 30 min
1000-1873K for 4040 s
1000-1900K for 4790 s
Ignition tests:
5.0 kW for Phase-1 and 15.0 kW for
Phase-2
1173-2013K for 1489 s
Constant 1.25 kW for 500 s
1.6-3.3 kW for 1000 s
As summarized above, the air oxidation tests have been performed from the separate to integral effects tests with zirconium based alloy cladding. From these tests, attention has mostly focused on the accelerated oxidation due to the presence of nitrogen, rather than the nitrogen uptake itself. Nitrogen does not react significantly with zirconium unless there is already some oxygen uptake, and that nitriding uptake is small if oxygen is present. For this reason, it was not though important until recently.
Furthermore, the re-oxidation of nitride has received little attention in the nuclear field.
Based on the past studies cited in this chapter, several types of air oxidation model have been developed. A summary of the current status of air oxidation development is given in Table 1.3, which shows that current Zr-air reaction models are mostly aimed to address the effect of nitrogen as a catalyst for the oxidation kinetics.
7
Table 1.3 Air oxidation model development for reactor system analysis codes MELCOR
SCDAP/RELAP5 MAAP ICARE
-CATHARE ATHLET-CD SOCRAT
Controlling
parameter Oxide thickness Weight gain O2 and N2 Diffusion rate in o
xide layer Reactant
gas O2 O2
Pre-
transition Parabolic kinetic rate Diffusion based
Transition initiator
Critical thickness is reached and
if nitrogen is present
t-ZrO2 to m-ZrO2
phase transformationf
Post-
transition Linear/accelerated kinetic rate Linear Enhanced diffusion coefficient
Role of
nitrogen Catalyst
ZrN formation reaction rate
under very low oxygen partial pressure
Loss of coherent microstructure
modelled by enhancement of oxygen diffusion
coefficient
PSI breakaway oxidation model is implemented into MELCOR code 1.8.6 version through the CORPSI record and MELCOR code 2.1 version through the COR_OX.
MAAP air model considers a porosity development of the oxide scale by the formation of nitride and its re-oxidation.
ICARE-CATHARE air oxidation model, the breakaway transition is considered by taking into account of transformation of phases from tetragonal ZrO2 to monoclinic ZrO2. In addition, an Air oxidation model for the ICARE-CATHARE code was developed by taking into account the nucleation-growth of ZrN (based on the Mampel model) but N2 is still considered as a catalyst.
In ATHLET-CD, the ZrN formation is considered when the ratio of oxygen partial pressure and total pressure decreases between 10−2≥ 𝑃𝑂2
𝑃 ≥ 10−3 to simulate the oxygen starvation condition.
8
In the SOCRAT air oxidation model, nitride formation was modeled by modulating the diffusion coefficient in the mixture of oxide and nitride layer. The diffusion coefficient of oxygen is enhanced due to the porous layer which is formed by the volume mismatch between the oxide and nitride layer.
As mentioned above, the current modeling of Zr-air reaction only considers the role of nitrogen as a catalytic effect by promoting the reaction kinetics. However, the nitrogen is not a catalyst, and it forms the ZrN and possibly other nitrogen containing phases.
In order to improve the current modeling of Zr-air reaction, a better understanding on the mechanism of nitriding and re-oxidation needs to be achieved by a systematic designed experiments and in-depth analyses that the present thesis aims. The current state of the art for nitriding and re-oxidation is provided in chapter 1.2.3 and research questions are proposed in chapter 1.3.
9
1.1.2.2 Past studies for a fundamental knowledge of Zr-O-N system and for more general application
In the past, several experimental works were performed to understand the behavior of nitride in presence of oxygen. Fig 1.1 summarizes the past studies related to the Zr- O-N system chronologically.
Fig 1.1 Chronology of the past studies related to the Zr-O-N system
10
Firstly, Dravnieks [45] reported the increase of the rate of nitrogen uptake into a Zr metal when oxygen partial pressure is sufficiently low. This provides the clue of favorable conditions of nitrogen incorporation and nitride formation. On the other hand, Hayes [46] reported the instability of ZrN in the presence of incoming oxygen and its decomposition to a voluminous oxide. It indicates the re-oxidation of ZrN by oxygen.
Furthermore, Nelson [47] burned the partially nitrided zirconium in pure oxygen atmosphere and observed explosions of nitrided zirconium. This indicates high exothermic nature of the re-oxidation of nitrided zirconium. In addition, Maekawa and Ishii [48] reported the instability of absorbed nitrogen and emphasized the importance of study of the ternary Zr-O-N system. Hence, Gilles et al. [49] firstly reported the structural data of several oxynitride phases. Subsequently, Collongues et al. [50]
studied the Zr-O-N system and reported that zirconium oxynitride is an intermediate phase between oxide and nitride. These studies were performed more than a half century ago and they addressed the instability of soluble nitrogen in presence of oxygen and its high exothermic nature. Moreover, one of oxynitride phase (Zr2ON2) was observed by X-ray diffraction analysis by Nelson [47] and the Zr-O-N system was studied as a solid solution of oxide and nitride by Collongues et al [50].
However, the nitrogen incorporation into the Zr-O system was not investigated in these earlier studies. Evans et al. [51] firstly reported that nitrogen is responsible for monoclinic to cubic ZrO2 conversion.
Afterward, Claussen [52] stabilized cubic ZrO2 from monoclinic ZrO2 in N2 at 1700°C.
After Claussen’s finding, Tendeloo and Thomas [53-54] formed a distorted cubic fluorite structure of ternary phase (Zr7O11N2) from a mixture of monoclinic ZrO2 and ZrN in N2 at 1900°C. In addition, Ikeda et al. [55] reported a new face centered cubic phase, oxygen stabilized ZrN(O) in monoclinc ZrO2 and ZrN in N2/H2 at 1600°C. Above findings were the stabilization of cubic ZrO2 and several types of oxynitrides from the reaction between monoclinic ZrO2 and ZrN. On the other hand, Cheng and Thomson [56] reported the formation of nitrogen-stabilized cubic ZrO2 from tetragonal ZrO2
above 1700°C in N2. Also, Delachaux et al. [57] reported a nitriding of tetragonal ZrO2. After then Lerch and his colleagues intensively studied the Zr-O-N system [58-61] and published several papers regarding the synthesis and structure [62-65], kinetics and thermodynamics [66-67] and diffusion behaviors [68]. Finally, Lerch summarized all
11
his findings regarding the Zr-O-N system and behavior of nitrogen in the ternary system [69]. In addition, the energetics of Zr-O-N system was also reported by Molodetsky [70-71] and the formation of intermediate phase (Zr-N-N-O-Zr) of the Zr- O-N system was reported by Wiame et al. [72]. Morever, Polfus [73-76] studied theoretically and experimentally nitrogen incorporation in oxide for the application of the Zr-O-N system into fuel cell fabrication and other electronic devices. Indeed, the Zr-O-N system has been recently studied for the application of electrical devices such as thin film in semiconductors (e.g. [77]) and fuel cell fabrications (e.g. [78]).
Although there have been several studies on the properties of the Zr-O-N system and its phases, studies on the ternary phase diagram have been quite scarce. Ermoline et al. [79] reported the ternary phase diagram of the Zr-O-N system at very high temperatures from 1200°C even to 2700°C. In their study, they used powder samples of Zr, ZrO2 and ZrN and heated the samples by CO2 laser beam. They suggested tentative subsolidus phase relations in Zr-O-N system above 1200°C. However, no investigation was achieved on the various solid solutions of the Zr-O-N system and the oxynitride phases. Their study is only valid above the oxynitride decomposition after the melting. In addition, Do et al. [80] reported the ternary phase diagram of Zr- O-N only focusing on the Zr-rich corner, where the oxygen partial pressure is very low.
They achieved the equilibrium oxygen partial pressure around 10-11 atm using a Mo/MoO2 redox couple at 1200°C. They reported the Zr-rich corner of ternary phase diagram of Zr-O-N system with α-Zr, ZrO2 and Zr(N,O)1-x which is the oxygen containing ZrN phase at 1200°C.
As explained above, the studies of zirconium reaction with oxygen and nitrogen has been performed not only in the nuclear field but also in the non-nuclear field. A more detailed explanation of the systems Zr-O, Zr-N and Zr-O-N is given in chapter 1.2.2.
12
1.2 Basic Knowledge
In this chapter 1.2, the basic knowledge is summarized for the metal oxidation reaction and its kinetics in chapter 1.2.1 and the zirconium metal oxidation and nitriding in chapter 1.2.2.
1.2.1 Metal oxidation reaction and its kinetics
Hilton [81] and Wagner [82] summarize the metal oxidation. This chapter summarizes the metal oxidation based on [81-82].
The Gibb’s free energy, G, is the thermodynamic driving force to lead the most stable reaction product by the largest negative standard free-energy change, △G. Metal oxidation is usually thermodynamically favored due to its negative △G in the course of the simultaneous combined oxidation and reduction reactions. Elemental metal atoms lose electrons when they react with molecules or atoms of oxidizing agents which receive electrons from atoms of the metal. The metal that loses electrons is oxidized and the species that obtain the electrons are reduced. The transfer of electrons is “counted” in terms of Oxidation Number, which increases from 0 for elemental Zr to +4 for ZrO2. The oxygen or steam could be oxidizing species. The reaction to produce nitride also increases the oxidation number, and in that sense nitrogen can be thought as a generalized oxidizing agent (though a weaker one than oxygen). For example, Fig 1.2 illustrates the oxidation reaction between metal and oxygen.
13
Fig 1.2 Schematic illustration of metal oxidation (excerpted from [81])
As shown in Fig 1.2, the oxidation process is described by three mechanisms. First, the oxygen molecules are adsorbed (O2 ads in Fig 1.2) on the metal surface. Second, the oxygen molecules are dissociated and ionized by electrons from metal and metal ions are formed when it loses electrons. The following equation from [81] describes the net oxidation reaction between metal and oxygen (M: metal and z: valence).
Oxidation: M → Mz++ ze−
Reduction: z
2O2+ ze− →z
2O2−
Combined: M +z
2O2 → MOz
2
Finally, metal cations and the oxygen anions are incorporated and a growing oxide film is formed. The oxide film growth is continued not only by the electric field between oxygen ions and metal ions but also by the diffusion due to a concentration gradient.
As the oxide layer grows, the electric potential difference decreases and also the concentration gradient decreases. For this reason, the oxide layer on the metal surface acts as a diffusion barrier, and thus improves the corrosion resistance by acting as a protective layer.
A stress builds up in the oxide layer by the different volume of the oxide and metal structures. This size mismatch causes the compressive hoop stress. At first, the compressive hoop stress results in a tighter inter-molecular microstructure of the oxide,
14
which resists more strongly the diffusion of oxygen through it. The reduced diffusivity results in kinetics that is approximately cubic instead of parabolic. However, as the oxide layer becomes thicker, the stress increases further, producing cracks or pores in the oxide. Then the oxide layer loses most of its protectiveness. Through the porous and cracked oxide layer, oxidizing species (e.g. oxygen or steam) intrude and the oxidation reaction occurs linearly, in other words, the resistance to oxidation is no longer increased with oxide thickness and the rate reaches a constant value or might possibly decrease. This phenomenon is referred to as breakaway.
Metal oxidation reaction kinetics is presented by the rate of reaction and the time. The rate of reaction is presented in oxide thickness, which is equivalent to mass gain (i.e.
oxygen mass gained during oxidation by oxygen or steam) [81].
A power-law expression is used to describe the metal oxidation reaction [81]. This is written as y = k ∗ tn (1).
In equation (1), y is for instance the mass gain per unit surface area and k is the reaction rate that is normalized by surface area because the reaction rate is proportional to the surface area, and t is the time and n is the exponent that indicates the kinetic model.
i) Parabolic kinetics (n = 0.5) y = kp∗ t
1 2 (2)
According to Wagner [82], the parabolic rate law is valid as long as the reaction kinetics is determined by the diffusion of oxygen anion or metal cations through the growing oxide scale. The parabolic time dependence describes the oxide layer grows fastly at first but slowly grows with time as the oxide grows. In the parabolic kinetics, the reaction rate decreases with time as the oxide grows [81]. As previously mentioned, the reaction rate of Zr alloys can be decreased to the cubic kinetics at temperatures below 1000°C.
ii) Linear kinetics (n = 1) y = kl∗ t (3)
The reaction rate maintains constant value with time. In addition, linear kinetics could be represented by a series of parabolic kinetics, in other words, pseudo-linear kinetics as shown in Fig 1.3.
15
Fig 1.3 Pseudo-linear oxidation kinetics (excerpted from [81])
As shown in Fig 1.3 (a), the oxide grows according to the parabolic kinetics until the certain thickness. After the reaching certain oxide thickness, oxide becomes degraded by crack formation and the oxidant can be transported close to the metal surface as a gas phase with a much shallower depth for diffusion. After then, new oxide layer grows on the metal surface. This repeated parabolic oxidation and spallation gives the pseudo-linear kinetics as shown in Fig 1.3 (b) [81].
iii) Para-linear (n = 1 and 0.5) 1
𝑦= 1
𝑘𝑙∗𝑡+ 1/(𝑘𝑝∗ 𝑡2 1) (4)
The para-linear oxidation is the combination of parabolic and linear kinetics.
Fig 1.4 Para-linear oxidation kinetics (excerpted from [81])
16
As shown in Fig 1.4, oxygen gas can penetrate readily the external porous layer to reach the metal or a protective oxide layer. After then, oxygen anions diffuse through the inner protective oxide layer to the metal-oxide interface. External porous layer causes the linear kinetics while the inner protective layer results in the parabolic kinetics [81].
The dependence on temperature of the coefficient of rate of reaction, k is described by the Arrhenius equation,
k = k0exp [−Q
RT] (5)
where k0 is the pre-exponential factor and Q is the activation energy for oxidation reaction in kJ/mol and R is the gas constant, 8.314 J/mol/K. The Arrhenius equation has been widely used to model thermally-induced reactions like the oxidation.
In this chapter, the basic knowledge on the metal oxidation reaction is provided.
However, experiments performed in the present thesis use the zirconium alloy cladding, Zircaloy-4. It is noted that the alloying elements have only minor effect on high temperature oxidation compared to the pure zirconium. The details of Zircaloy-4 sample will be given in chapter 2.1.4.
17
1.2.2 Zr-O, Zr-N and Zr-O-N systems
There are several phases in the Zr-O-N system possibly involved in oxidation (also in re-oxidation) and nitriding process. Table 1.4 provides the most relevant properties of all known phases in the Zr-O, Zr-N and Zr-O-N systems.
Table 1.4 Summary of the Zr, Zr-O, Zr-N and Zr-O-N system
System Phase Structure Density
[g/cm3]
Molar volume [cm3/mol Zr]
Zr metal
α-Zr Hexagonally close
packed (hcp) 6.52 13.99
β-Zr Body centered
cubic (bcc) 6.41 14.23
Zr-O binary system
m-ZrO2 Monoclinic 5.68 21.14
t-ZrO2 Tetragonal 6.10 20.20
c-ZrO2 Cubic fluorite
structure 6.09 20.23
Zr-N binary system
o-Zr3N4ᵃ Orthorombic 6.34 17.33
c-Zr3N4ᵇ Cubic 7.16 15.35
ZrNᶜ,ᵈ* FCC B1 (NaCl)
rock salt structuree 7.09f 14.84
Zr-O-N ternary system
β’-Zr7O11N2 Rhombohedralg 6.05h 19.90 β’’-Zr7O9.5N3.0
Irregular succession of β phase and m-ZrO2i
n/a n/a
β-Zr7O8N4 Rhombohedralg n/a n/a
γ-Zr2ON2* cubic bixbyte
structurei,k 5.77 19.63
ᵃ[60]
ᵇ[83]
ᶜ[84]
ᵈ[85]
e[86]
f[87]
g[55]
h[88]
i[89]
k[90]
*ZrN and γ-Zr2ON2 are optically golden-yellow color [91].
18
In the next sections, zirconium metal is described firstly and then the two Zr-O and Zr- N binary systems are described. Lastly, the Zr-O-N ternary system is discussed.
1.2.2.1 Zirconium metal
At room temperature, zirconium has a hexagonally close packed (hcp) crystal structure α-Zr and allotropically transforms to a body centered cubic crystal structure β-Zr at 867°C and remains in β-Zr until the melting point 1855°C. In the Zr-O binary system, α-Zr dissolves oxygen up to 30 at.% (7.0 wt.%) forming the oxygen stabilized α-Zr(O) (see Fig 1.5). In the same way, α-Zr dissolves nitrogen up to 22 at.% (4.2 wt.%) forming the nitrogen stabilized α-Zr(N) in the Zr-N binary system (see Fig 1.6). The nitrogen reacts significantly with α-Zr(O) but it barely reacts with β-Zr [28]. It is likely that an oxygen/nitrogen stabilized α-Zr(O,N) phase would be formed as follows: Zr + x
2O2 +
y
2N2 → α-Zr(Ox, Ny) [28].
1.2.2.2 Zirconium oxide phases
The oxygen saturated α-Zr is in equilibrium with the oxide phases.
Fig 1.5 Zr-O phase diagram (excerpted from [92])
19
As shown in Fig 1.5, there are three different zirconium oxide phases on the oxygen- rich side of phase diagram with monoclinic, tetragonal and cubic structure with increasing temperature. The maximum sub-stoichiometry of the oxide in equilibrium with α-Zr(O) and the liquid metal, respectively, increases from monoclinic via tetragonal to cubic phase, which influences the oxygen diffusion coefficients in the oxide.
Furthermore at temperatures below 1050°C, the oxide layer may contain a significant amount of tetragonal (t-ZrO2) where m-ZrO2 should be thermodynamically stable in its temperature range [93].
The oxide scale is developed mainly by m-ZrO2, but a small quantity t-ZrO2 is formed close to the metal/oxide interface as a very thin layer [94]. The tetragonal phase is preferentially stabilized near the interface by some of the following reasons [93] and [95-96].
1. High compressive stress in the protective oxide layer 2. Low grain size
3. The presence of defects; hypo-stoichiometry.
With progressing oxidation, these three effects are reduced and the oxide transforms to its stable monoclinic structure. This is one of the explanations for breakaway oxidation.
Cubic and tetragonal phases can be stabilized by aliovalent ions down to room temperature. This effect is used for technical ceramics by adding oxides with cations of lower valency (e.g. MgO or Y2O3 to ZrO2). The same effect could be observed by adding anions with higher valency like oxygen, as it is the case for nitrogen, stable [66- 67] and [97].
1.2.2.3 Zirconium nitride phases
The nitrogen saturated α-Zr is in equilibrium with the nitride phase as shown in Fig 1.6.
Only metallic ZrN phase is shown in the nitrogen-rich side of phase diagram.
20
Fig 1.6 Zr-N phase diagram (excerpted from [92])
However, zirconium nitrides have complex electronic bonding systems. Due to the moderate difference in the electronegativity difference between zirconium (1.33) and nitrogen (3.04), zirconium nitrides have a mixture of ionic, covalent, and metallic bonding systems. From 2.0 to 4.0 of the value of electronegativity difference between two elements, ionic bond is developed. The ionic-covalent bond is established from 0.4 to 2.0 and the covalent bond is established from 0.0 to 0.4. Between zirconium (1.33) and nitrogen (3.04), the value of electronegativity difference is 1.71 and thus it forms the ionic-covalent bond, whereas in the case of zirconium (1.33) and oxygen (3.44), the electronegativity difference is 2.11, thus the ionic bond is formed.
It seems that the formation of ionic-covalent (polar bonding) zirconium nitride, Zr3N4, might be preferable than the formation of metallic zirconium nitride, ZrN, due to the electronic bonding between the zirconium and nitrogen atoms. The structure of the lowest energy for Zr3N4 is the orthorhombic at ambient pressure [90] and [98-99].
Zr3N4 is a metastable phase [90], [98], [100] and [101] and hence is thermodynamically unstable. Therefore, as the temperature increases Zr3N4 (Zr oxidation state: +4) loses its excess nitrogen and is converted to the mononitride ZrN (Zr oxidation state: +3) [102] with the formation of nitrogen vacancies (VN′′′) [90] as follows: Zr3N4 + VN′′′ ⟶ 3ZrN + N . Decomposition of Zr3N4 into ZrN and nitrogen gas was observed at
21
various temperature ranges: at 600°C by Camellio et al. [101] and at 800-900°C by Lerch et al., [62] and at 1000°C by Juza et al. [103]. In summary, the Zr3N4
decomposes at elevated temperatures as follows: Zr3N4 → 3ZrN + ½ N2 [50].
1.2.2.4 Zirconium oxynitride phases
The zirconium oxynitrides are stabilized at the high oxidation state (+4) of Zr [90] and at the oxidation states of the oxygen (-2) and nitrogen (-3), respectively. For this reason, the zirconium oxynitride phases are considered the intermediate phases between the ZrO2 (+4 Zr oxidation state and -2 O oxidation state) and Zr3N4 (+4 Zr oxidation state and -3 N oxidation state) [50] and they are described with general formula of ZrO2−2xN4x
3
Vax
2
[58]. For this reason, the stoichiometry of the zirconium oxynitrides appropriates to the system of ZrO2 with Zr3N4 instead of ZrN [90]. Indeed, the Zr-O-N ternary phases were described in the ZrO2-Zr3N4 solid solution according to the Zr3N4
mol fraction in [61] and [70] as shown in Table 1.5.
Table 1.5 Compositions of zirconium oxynitrides [70]
𝐙𝐫𝐎𝟐−𝟐𝐱𝐍𝟒𝐱 𝟑
𝐕𝐚𝐱
𝟐 Formula Zr3N4 mole fraction (x) β’ (𝑥 = 3
14) Zr7O11N2Va0.75 0.214
β’’ (𝑥 ~ 4.8
14) ~ Zr7O9.5N3.0Va1.2 ~ 0.343 β (𝑥 = 6
14) Zr7O8N4Va1.5 0.418
γ (𝑥 = 3
4) Zr2ON2Va0.75 0.75
Zirconium oxynitride phases retain the cubic fluorite structure of c-ZrO2 [52]. Due to the oxygen vacancies (Va) formed by the nitrogen incorporation, the original cubic lattice is gradually distorted. Firstly, these oxygen vacancies are distributed randomly but beyond a critical nitrogen concentration, the oxygen vacancies tend to be ordered [104]. Before transforming to the γ-Zr2ON2 phase, the c-ZrO2 contains the incorporated nitrogen with oxygen vacancies up to the lattice parameter a = 5.118 Å from originally 5.10 Å [56]. γ-Zr2ON2 has also a cubic bixbyite-type crystal structure which is close to
22
the cubic fluorite structure of c-ZrO2 [90] and its lattice parameter is almost twice (10.4303 Å) of c-ZrO2. β-type phases have rhombohedral structures that are slightly distorted from the original cubic lattice [53]. Both γ-Zr2ON2 and β-type structures have ordered oxygen vacancies [58]. In summary, zirconium oxynitride phases are structurally derived from the cubic fluorite structure of c-ZrO2.
In addition, Ohashi et al. [105] prepared the oxynitride phases by heating a mixture of m-ZrO2 and β-ZrNCl at 900-1000°C, and identified the Zr-O-N phases according to the O/Zr ratio as provided in Table 1.6.
Table 1.6 Zr-O-N phases according to O/Zr ratio [105]
O/Zr ratio range Zr-O-N Phases
O/Zr ratio ≤ 0.40
γ-Zr2ON2 and ZrN ZrN content increases with the decrease of O/Zr ratio 0.40 ≤ O/Zr ratio ≤ 1.00 solid solution of γ-Zr2ON2
1.00 ≤ O/Zr ratio ≤ 1.50 β-type phases O/Zr ratio ≥ 1.50
β-type phases and m-ZrO2
m-ZrO2 content increases with the increase of O/Zr ratio
As already mentioned in chapter 1.1.2.2, the past studies on the ternary phase diagram of Zr-O-N are very limited. Tentative subsolidus phase relations in the Zr- ZrO2-Zr3N4 ternary system were suggested by Ermoline et al. above 1200°C as seen in Fig 1.7 [79].
23
Fig 1.7 Tentative subsolidus phase relations in the Zr-O-N system (excerpted from [79]) However, the suggested ternary phase diagram is only valid at temperatures above the oxynitride decomposition by melting [79].
In addition, the Zr-rich corner of ternary phase diagram of Zr-O-N system at 1200°C was reported by Do et al. [80] as seen in Fig 1.8.
Fig 1.8 Zr-rich corner of ternary phase diagram of Zr-O-N system at 1200°C (excerpted from [80])
24
The major findings from studies of Do et al. [80] on the Zr-rich corner of the Zr-O-N system are the stability domains of Zr(N,O)1-x and α-Zr. It was reported that the ratio of (N+O)/Zr is almost unity in accordance with the boundary of Zr(N,O)1-x in equilibrium with ZrO2. It is noted that Zr(N,O)1-x is the oxygen containing ZrN phase and its ratio of (O+N)/Zr is below 1. Do et al. [80] also observed that the optical color of Zr(N,O)1-x
was yellow. In addition, Do et al. [80] found that the stability domain of α-Zr is considerably extended under the dissolution of both nitrogen and oxygen.
So far there has been two ternary diagrams of Zr-O-N system. However, they include no oxynitride phases and no ionic-covalent nitride phase, Zr3N4. Thermodynamic databases for Zr-O-N system are not developed, yet. For the Thermo-Calc calculation, there is only one Zr-based alloys database which is the TTZR1 database [106].
However, this database includes no oxynitride phases and no ionic-covalent nitride phase and metallic nitride.
From the review of phases in the Zr, Zr-O, Zr-N and Zr-O-N systems, the most important findings for an interpretation of results and a discussion of reaction mechanism are summarized as follows:
t-ZrO2 could be stabilized in the temperature range where m-ZrO2 is thermodynamically stable (below 1205°C; i.e. practically seen below 1050°C) due to high compressive stress in the protective oxide layer, small grain size and sub-stoichiometric oxide.
c-ZrO2 could be stabilized from t-ZrO2 in temperature range where m-ZrO2 is thermodynamically stable (below 1205°C) by incorporating the nitrogen.
Stability domain of α-Zr could be significantly extended when both oxygen and nitrogen are dissolved in Zr.
According to the ternary phase diagrams, there is an only low solubility of nitrogen in the oxide, but a considerable solubility of oxygen in the nitride phase.
A metastable Zr3N4 seems to be formed firstly before forming ZrN. As temperature increases, it loses its excess nitrogen and is converted to the metallic mononitride ZrN.
25
Zirconium oxynitrides are the solid solution of ZrO2 with Zr3N4 rather than ZrN.
All zirconium oxynitrides are structurally derived from the cubic fluorite structure of c-ZrO2
•
ZrN and γ-Zr2ON2 are optically golden-yellow color. For the past researches performed introduced in chapter 1.1.2.1, the golden-yellow inclusion was considered only ZrN. However, it could be mixture of ZrN and γ-Zr2ON2.26
1.2.3 Zirconium oxidation and nitriding
During air oxidation of zirconium, both oxidation and ntiriding occur. The overall process of air oxidation of zirconium is summarized in [107] as follows.
(1) O2 + Zr → ZrO2 and/or xO2 + 2Zr → 2Zr(Ox): zirconium reacts with oxygen and forms the zirconium oxide and/or α-Zr(O) absorbs the oxygen. The dissolved oxygen can stabilize the α-Zr(O) at high temperature until the melting temperature.
(2) O2 + 2ZrO + 2Vo(2+) + 4e- → 2ZrO2: in the oxide layer, oxygen anions diffuse to the metal and the oxygen vacancies move to the oxide surface to react with additional oxygen in air. After oxygen is sufficiently decreased, nitrogen can react.
(3) ½ N2 + 2ZrO + 2Vo(2+) + 4e- → ZrN + ZrO2: nitrogen reacts with zirconium through the previously developed oxygen vacancies in the oxide and forms ZrN (i.e. 1/2N2 + Zr → ZrN)
(4) ZrN + O2 → ZrO2 + ½ N2: ZrN is oxidized by newly-incoming oxygen and forms zirconium oxide and nitrogen gas is released.
The heat is released during air oxidation. The reaction enthalpies of each step at room temperature (25°C) are given in the following.
• Zirconium oxidation by oxygen: O2 + Zr → ZrO2 △Hrxn = -1094.8 kJ/mol Zr
• ZrN formation: ½ N2 + Zr → ZrN △Hrxn = -365.4 kJ/mol Zr
• ZrN re-oxidation: ZrN + O2 → ZrO2 + ½ N2 △Hrxn = -729.4 kJ/mol Zr
As above mentioned, nitrogen reacts with zirconium when oxygen is sufficiently consumed in the gas phase. The PO2/PN2 diagram which indicates the stability regions of Zr, ZrO2 and ZrN is calculated in [28] as seen in Fig 1.7. The stability diagram shows
27
that ZrN is stable only at very low oxygen partial pressure. Therefore, ZrN is formed under a locally very low oxygen partial pressure after a sufficient consumption of oxygen [28].
Fig 1.7 PO2/PN2 stability diagram (excerpted from [28])
From the result of separate effect tests performed by Steinbrueck et al. [28], nitrogen minimally reacts with pure zirconium and β-Zr(O). However, nitrogen readily reacts with α-Zr(O) and sub-stoichiometric oxide when oxygen is almost fully consumed. The sub-stoichiometric oxide is ZrO2-x is in equilibrium with oxygen-stabilized α-Zr(O) according to the Zr-O phase diagram. Under this locally almost oxygen starvation condition, the nitrogen reacts with α-Zr(O) and sub-stoichiometric oxide.
In addition, pores are formed during ZrN formation due to a volume mismatch between ZrN and ZrO2 [16]. According to Duriez et al. [15], as the further oxidation occurs inwardly, the newly formed inner-oxide puts the formed ZrN outwardly and then the ZrN is embedded in the oxide. This process continues as oxygen from the newly- incoming air partially oxidizes the previously formed ZrN and forms the ZrO2 again while the nitrogen is released to the atmosphere or combine again with unoxidized Zr atoms near the interface where oxygen partial pressure is sufficiently low. Due to
28
different molar volumes of ZrN (14.8 cm3 and ZrO2 (21.7 cm3), it leads to 46.6%
(∆VZrN→ZrO2 /VZrO2 = 6.91/14.8) of volume increase in the region of ZrN oxidation occurred. Due to this significant volume expansion, the oxide scale experiences local stresses which lead to very porous oxide and cracks in the oxide allowing air to penetrate deeper as a gas deeper.
This nitride-assisted degradation is self-sustaining. Because, some amount of the generated nitrogen during the oxidation of ZrN would be trapped in oxide and it is available for further ZrN formation. Also, released nitrogen is available for further reaction with the oxygen stabilized α-Zr(O) phase under local oxygen starvation [27].
Fig 1.8 illustrates the oxide layer of Zircaloy-4 in air oxidation and it gives the information of mechanisms of ZrN formation and its oxidation.
Fig 1.8 Mechanism of air oxidation (adopted from [108])
Region 1: initially formed dense oxide ZrO2 – the most outer region: the dense and columnar oxide that was formed during pre-transition period. Through the imperfections in oxide scale, air accesses to the interface and oxidizes the metal. The oxygen is almost starved locally and nitrogen reacts with the oxygen stabilized α-Zr(O)
29 or sub-stoichiometric oxide.
Region 2: porous oxide after oxidation of ZrN – ZrN converts to ZrO2 region: ZrN is oxidized by newly incoming air and converted to oxide. Due to the significant volume increase in the oxide, cracks are formed by compressive stresses.
Region 3: ZrO2/ZrN mixture – ZrO2/ZrN mixtures are stable according to the stability diagram as shown in Fig 1.7.
Region 4: α-Zr(O) – At the oxide-metal interface, the oxygen is almost starved locally and nitrogen reacts actively with the oxygen stabilized α-Zr(O).
As explained above in this chapter, the current understanding on the oxidation and nitriding is still rather empirical and phenomenological. It is based on the Zr-O binary system and partly on the Zr-N binary system as well on the P(O2)/P(N2) stability diagram. For this reason, only binary compounds, ZrO2 and ZrN, are involved in the oxidation and nitriding process. No ternary phases have been considered in the mechanism of oxidation and nitriding the past studies, except the mutual solubility in the metal phase as well of O in ZrN and N in ZrO2. However, this mutual solubility limit was not reported.
However, it is possible that the golden-yellow inclusions as shown in Fig 1.8 could be a mixture of γ-Zr2ON2/ZrN. Since both γ-Zr2ON2 and ZrN optically have a golden-yellow color [91] and the metastable phase of Zr3N4 may decompose into both γ-Zr2ON2 and ZrN. For instance, Lerch [61] reported nitrogen rich part of Zr3N4/γ-Zr2ON2 phase decomposes to ZrN and nitrogen gas and β-phase at around 840°C as follows: Zr3N4
→ 3ZrN + ½ N2 (accompanied by -14.37% molar volume shrinkage from Zr3N4) and γ- Zr2ON2 → β-type oxynitrides + xZrN + yN2 (from γ-Zr2ON2) [50]. Dzivenko [83]
observed that firstly Zr3N4 is decomposed to γ-Zr2ON2 and further to m-ZrO2 at temperatures above 600°C as follows:
4 Zr3N4
+3O2−2N2
→ 6 γ-Zr2ON2
+9O2−6N2
→ 12 m-ZrO2;
In addition, Cheng and Thompson [104] reported γ-Zr2ON2 phase decomposed to the ZrN and β-type oxynitride phases above 1050°C.
By considering the oxynitrides and Zr3N4, the nitriding could be explained as follows.
30
• Nitriding product Zr3N4: ZrOx + ⅔ N2 → ⅓ Zr3N4 + Ox and/or Zr + ⅔ N2 → ⅓ Zr3N4
• Decomposition of Zr3N4: Zr3N4 → 3ZrN + N
• Nitriding product of Zr2ON2: ½ ZrO2 + ½ Zr3N4 → Zr2ON2
• Decomposition of Zr2ON2: Zr2ON2 → β-type oxynitrides + xZrN + yN In addition, the re-oxidation could be explained as follows.
• Re-oxidation of ZrN: O2 + ZrN → ZrO2 + ½ N2
• Re-oxidation of zirconium oxynitrides: xO2 + ZrO2−2xN4x
3
→ ZrO2 + 2x
3 N2
• Re-oxidation of Zr2ON2: Zr2ON2 + 3/2 O2 → 2ZrO2 + N2
In addition, the stress evolution by volume mismatches in the oxide and nitride (possibly oxynitride, as well) scale will be different according to the Pilling-Bedworth ratio (PBR).
PBR =volume of oxide and nitride produced
volume of metal consumed = Wd
nDw= W ∙ 6.52 n ∙ D ∙ 91.224 where,
W: molecular weight of oxide and nitride d: density of metal (Zr = 6.52 g/cm3)
n: number of metal atoms in the oxide and nitride
D: density of oxide/nitride/oxynitride (values are provided in Table 1.4) W: atomic weight of metal (Zr = 91.224 g)
The PBRs of Zr-O and Zr-N binary system are shown in Table 1.7 and it is calculated using above equation based on Table 1.4.
Table 1.7 PBRs of the Zr-O and Zr-N binary system
System Phase PBR
Zr-O binary system m-ZrO2 1.511
t-ZrO2 1.444
c-ZrO2 1.446
Zr-N binary system c-ZrN 1.061
31
A PBR of less than 1 results in tensile stress in the scale and compressive stress in the metal. On the other hand, PBR greater than 1 causes compressive stress in the scale and tensile stress in the metal.
From the Raman spectroscopy measurements of air-oxidized Zircaloy-4 samples by Idarraga et al. [19-20], Lasserre et al. [21-22] and Kadiri et al. [109], several phases were observed as follows. Idarraga et al. [19-20] reported the ZrN near the interface, and zirconium oxynitrides and c-ZrO2 close to the ZrN from the Zircaloy-4 air oxidation test in the 800-1000°C by Raman investigation. In addition, Lasserre et al. [21-22]
observed the estimated γ-Zr2ON2 signal in the Zircaloy-4 air oxidation test at 850°C and Kadiri et al. [109] reported the zirconium nitrides in form of oxynitrides from the Zircaloy-4 air oxidation test at 900°C by Raman investigation, respectively. However, all their Raman investigations to identify the oxynitride phases were not sufficiently proven with the reference oxynitride Raman spectrum, or with other complementary measurements.
Furthermore, from the results of post-test investigations (e.g. separate effect tests performed at IRSN [15-17] and performed at KIT [24-30]), it was not possible to observe the γ-Zr2ON2 phase since there were no detailed structural analysis during the oxidation tests (e.g. in-situ X-ray diffraction) to determine the γ-Zr2ON2 phase in the oxide layer. For this reason, only a ZrN layer was reported from their air oxidation tests.
For this reason, this PhD thesis also conducted the Raman spectroscopy measurements and analyzed its data with well-designed methodologies (in chapter 3.3.1) and with several analytical measurements (e.g. EDS, WDS, SEM, XPS and XRD in chapter 3.3.2) to investigate the possibility of oxynitride phase during nitriding and re-oxidation.