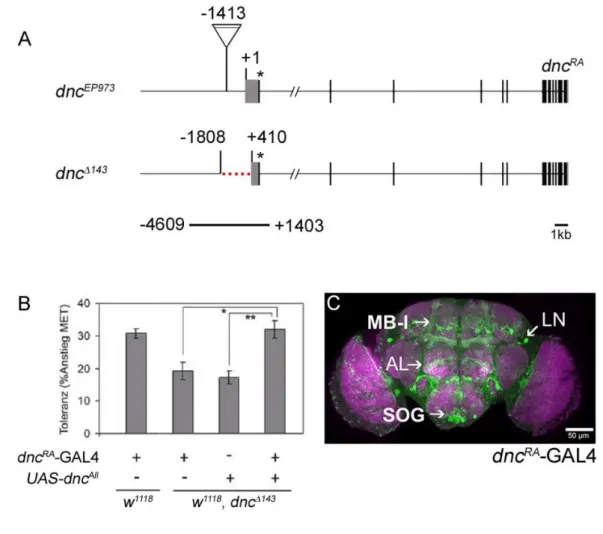
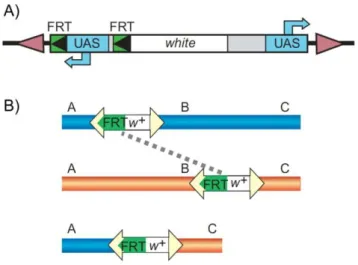
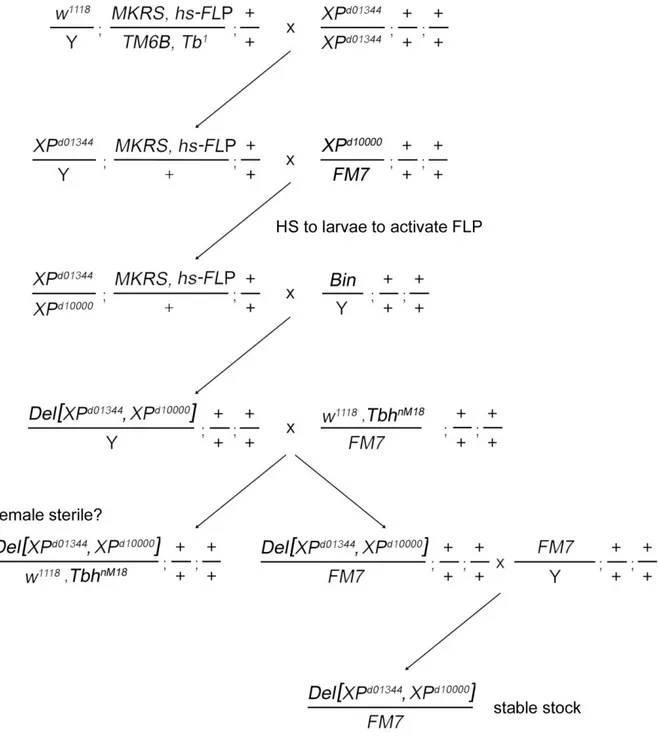
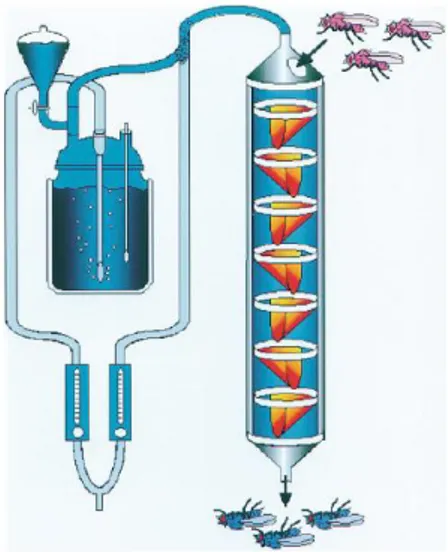
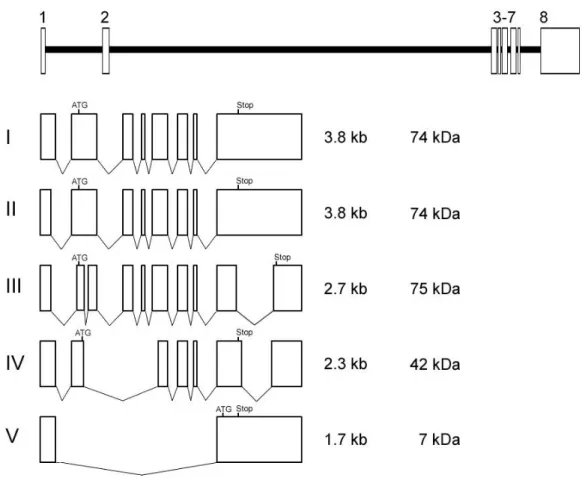
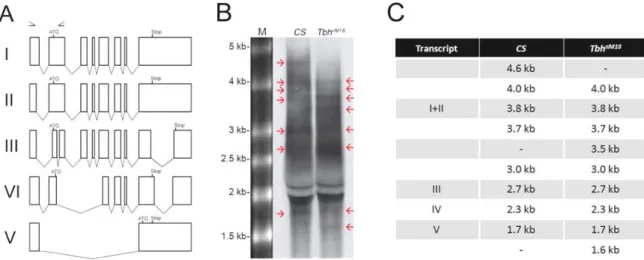
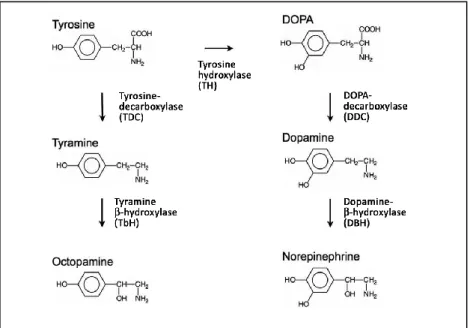
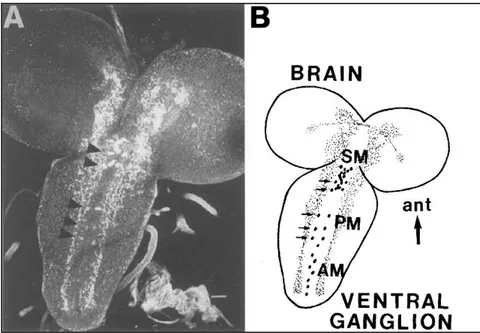
Dissecting Tbh and Hangover function in ethanol tolerance in Drosophila melanogaster
Volltext
Abbildung
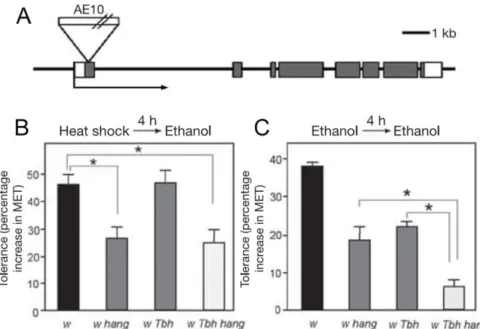
ÄHNLICHE DOKUMENTE
Confronting Antisemitism and Intolerance: The Second Conference 23 What kind of challenges and opportunities does Jewish- Muslim dialogue
This paper presents estimates of the global energy efficiency improvement potential by applying first- and second-law, or exergy, analysis to regional and
First and most common, inflation rates and economic growth rates are included besides share prices, dividends, and nominal capital as additional endogenous time series into the
• Palestine, Class 3 (15.4%) very often contrasts the representation (almost without exception) of cooperative behavior and (relatively frequently) its announcement with
SCB clitics can appear in either prosodically defined second position (after the first prosodic word) or in positions that can be characterized in syntactic terms (after the
Taken together, the loss of lost activity, an increased amount of osk RNA within the PGCs and maternal overexpression of the pgc 3’UTR all lead to the same
Auf die Hethiter übertragen heißt das: Erst wenn sich im hethitischen Reich viele an der Aufbringung der Opfer beteiligen, wiegen die Kosten (für die Opfer- gaben) den Nutzen (also
brochure (German, French, Italian) published by the Federal Food Safety and Veterinary Office FSVO. • Federal Food Safety and Veterinary Office FSVO flyer