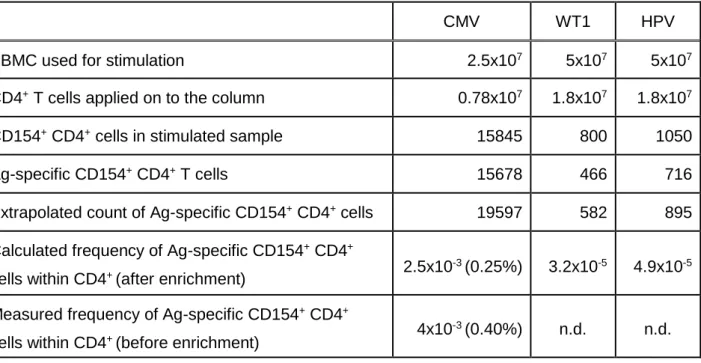
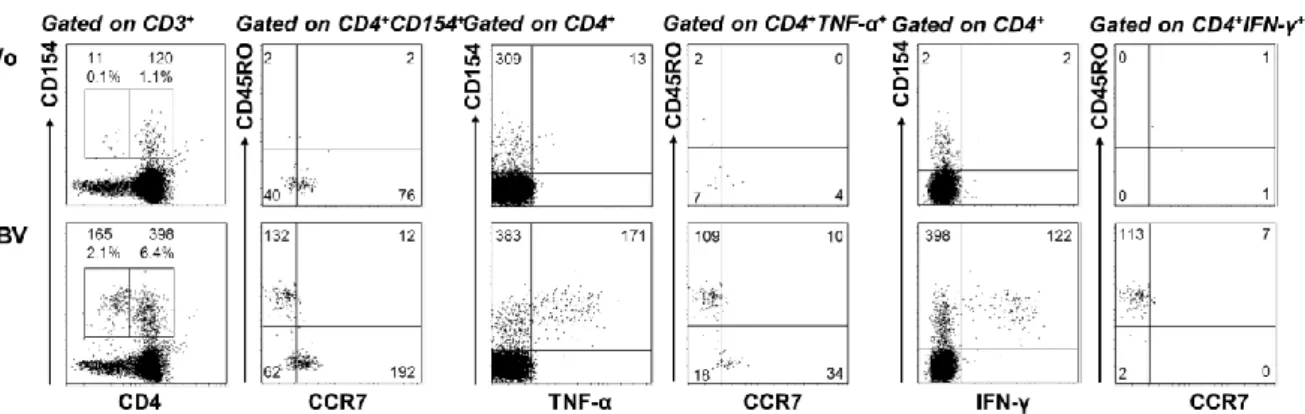
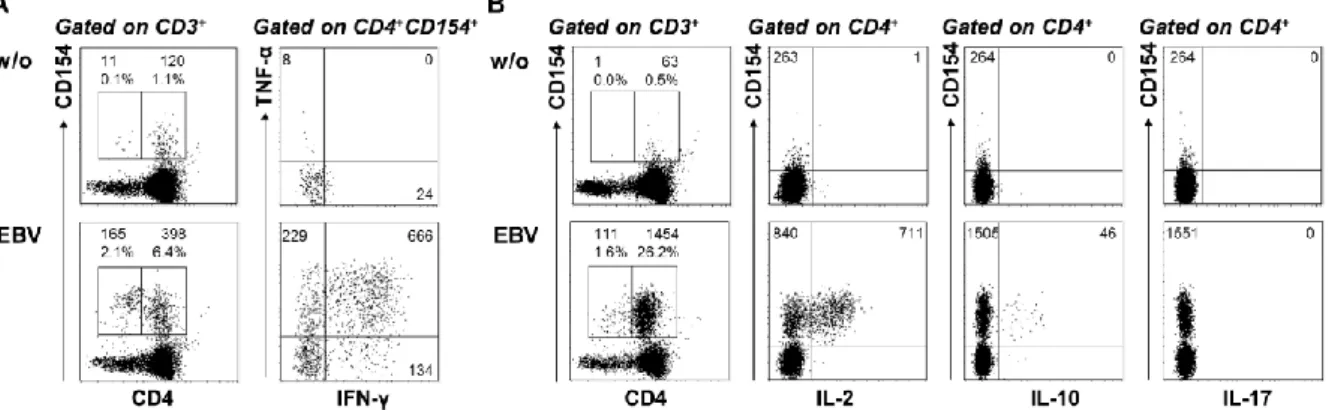
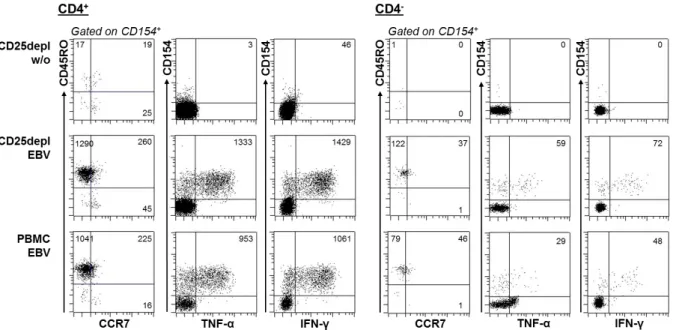
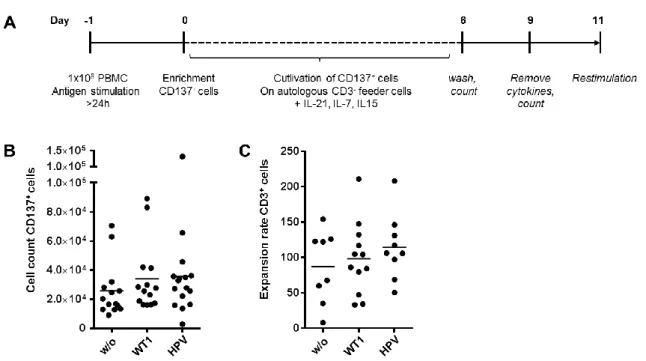
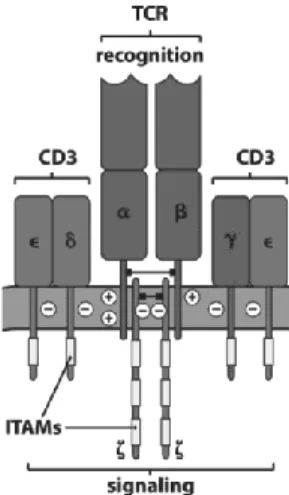
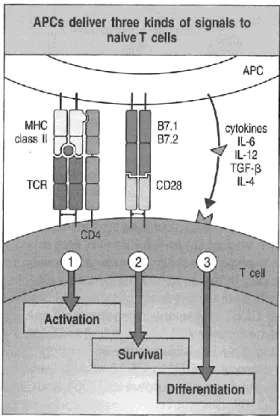
„Characterization of tumor-antigen-specific T cells – insights into WT1- and HPV16-specific T cell repertoires“
Volltext
Abbildung
ÄHNLICHE DOKUMENTE
[r]
To exclude that the reduced number of virus-specific T cells in Bis VIII–treated mice at day 7 after viral infection was due to inhibition of T cell activation and reduced expansion,
Among aged donors, statistical differences in basal glycolysis, compensatory glycolysis and the ratio basal mitoOCR/glycoPER between memory CD4 + T cells and naive CD4 + T. cells
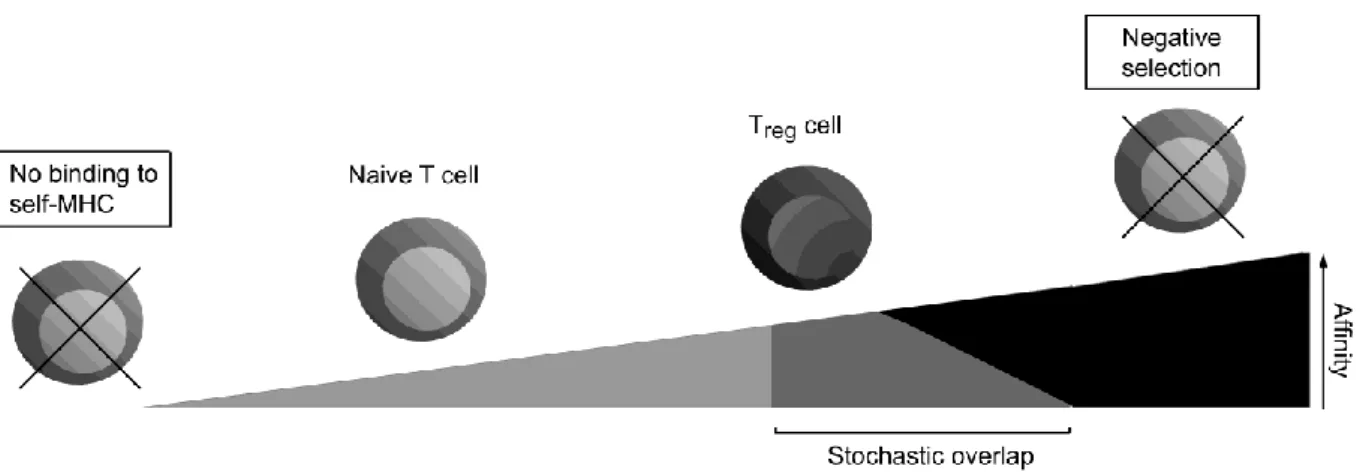
fewer# OT6I# T# cells# survive# negative# selection# in# RIP6variant# mice# expressing#
RESULTS: We show for the first time that EC present a quantitatively different peptide repertoire with abundance of certain peptides, compared with leukocytes. The abundance of
In a clinical trial, transferring CMV-specific T cell from the stem cell donor in CMV-reactivating HSCT recipients, patients with proliferating CMV-specific T
The cell-surface binding and internalization process of human papillomaviruses is a complex multi-step process that is not yet fully understood. To date, numerous
Here it is shown for the first time that in vitro conversion of CD4 + CD25 - T cells into CD4 + CD25 + Foxp3 + Tregs was significantly reduced in cells derived from