1
Repetition, Year 9, Term 2, Second Sheet
I) photon from a IR laser has less energy than a
A) microwave photon B) UV laser
C) radio wave photon D) none of the above
II) A photons from a red laser has more energy than a
A) IR photon B) UV photon
C) x-ray photon D) green photon
III) Fluor is the 9th element in the periodic table. In the ground state, the electronic structure of Fluor is hence
A) 1s22s22p5 B) 1s22s23s23p1 C) 1s22s22p23s23p1 D) 1s9
1) Calculate the photon energies associated with the transitions from the third energy level (n=3) to the ground level in both Hydrogen and Mercury using the energy diagram below:
2) Use the energy level diagram to state the ionization energy of Mercury in the ground state.
3) Calculate energy of the second energy level (n = 2) in Hydrogen.
4) Consider light with a frequency of
2.6× 10
14 Hz. Calculate the energy of each photon.Hint: Planck’s constant is 4.136×10−15eV⋅s .
2
5) The energy of a photon from a green laser is 2.6 eV, which corresponds to 4.0×10−19 J.
Consider a green laser with a power of 20 W. How many photons does this green laser emit each second, assuming that the efficiency of the laser is 30 %?
6) 2 3892U is an unstable isotope of Uranium that decays into and isotope of Thorium (Th) by alpha decay. Calculate A and Z for the daughter nucleus.
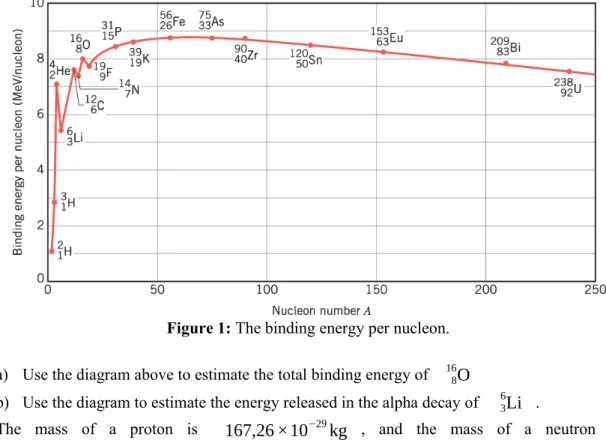
7) Take a look at the diagram in Figure 1 below:
Figure 1: The binding energy per nucleon.
a) Use the diagram above to estimate the total binding energy of 168
O
b) Use the diagram to estimate the energy released in the alpha decay of 36
Li
.8) The mass of a proton is
167,26
×10−29kg
, and the mass of a neutron is 167,49×10−29kg . The mass of the nucleus of 2656Fe is 9288 ×10−29kg . Calculate the mass defect of Fe-56.9) Calculate the binding energy of Fe-56 based on exercise 12.