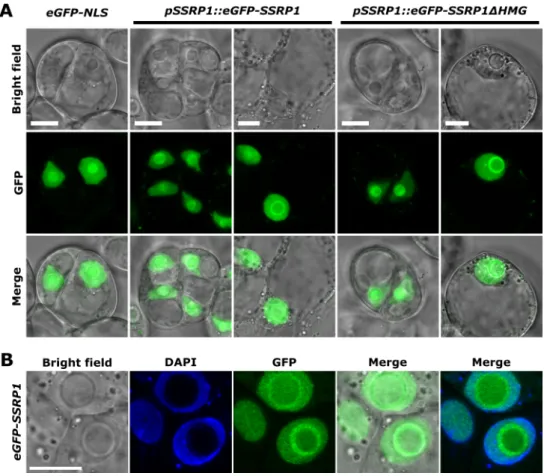
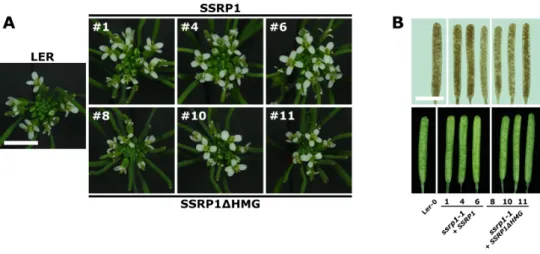
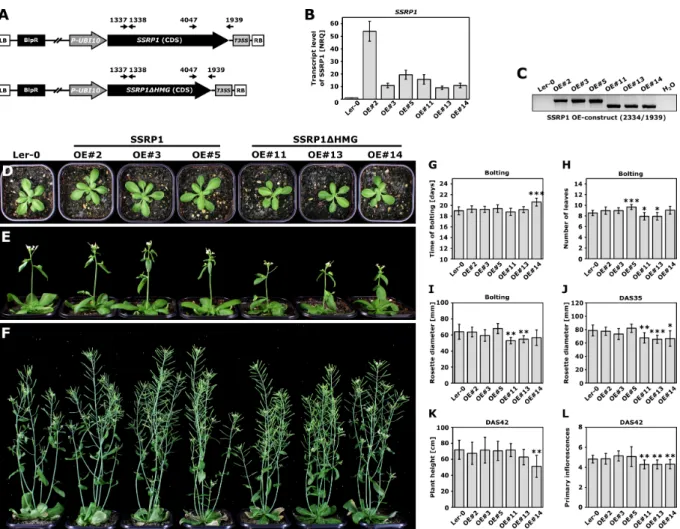
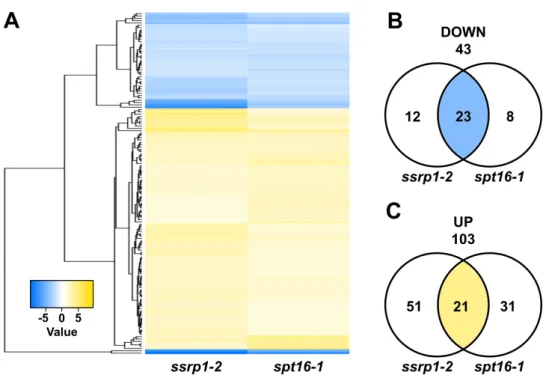
Molecular and functional studies on the transcript elongation factor FACT and the SAGA-DUBm subunit ENY2 in Arabidopsis
Volltext
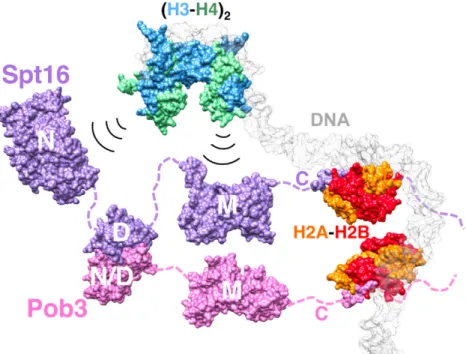
Abbildung



ÄHNLICHE DOKUMENTE
Vrtti and abhinaya are classified on the basis of the innate human
As we approached Walvis Ridge at the beginning of the week the petrologists attempted unsuccessfully to sample an isolated seamount flanking Walvis Ridge to the south.. Later in
First, RNAPII is recruited to the promoter, and then the general transcription factors bind RNAPII and initiate transcription (pre-initiation complex assembly,
When comparing chromatin of SPT4-R3 and Col-0 plants, increased levels of SPT5 and transcribing RNAPII (Ser5P, Ser2P) were found along the transcribed region of the long At3g02260
A rat glioma cell line (RuGli) adhered via α1ß1 integrin to collagen XVI (Eble et al., 2006), whereas human fibrosarcoma cells showed mainly interaction via predominantly
Feeding experiments with specifically 13 C-labeled glucose disclosed that the diterpenoid part of the striatals/striatins is formed via the mevalonate pathway, whereas the
fragment. Thus, these complexes react predominantly end-on by using the phosphorus lone pair. 4 In complexes of type B the lone pair is already used by coordination to Lewis
WITH THE POWER CONTROL MODULE ORIENTED AS SHOWN IN FIGURE 2, CAREFULLY ROUTE THE MODULE CONNECTORS THROUGH THE OPENING BETWEEN THE FAN HOUSING AND THE POWER SUPPLY BOARD.. THE