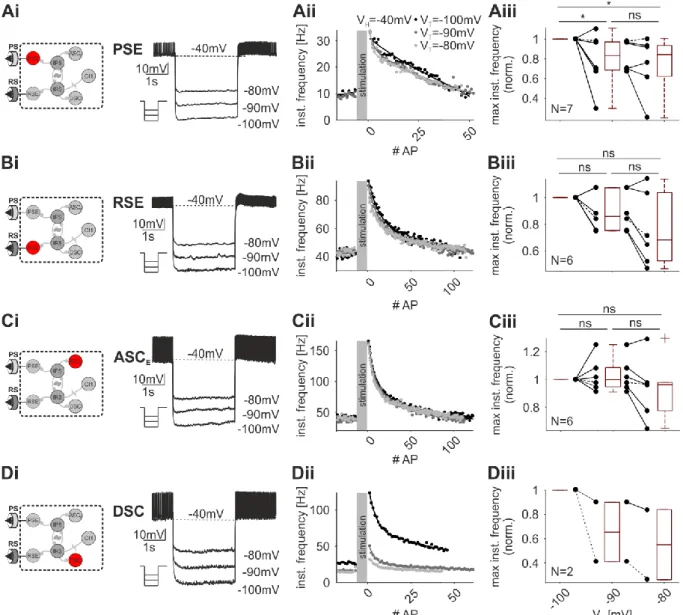
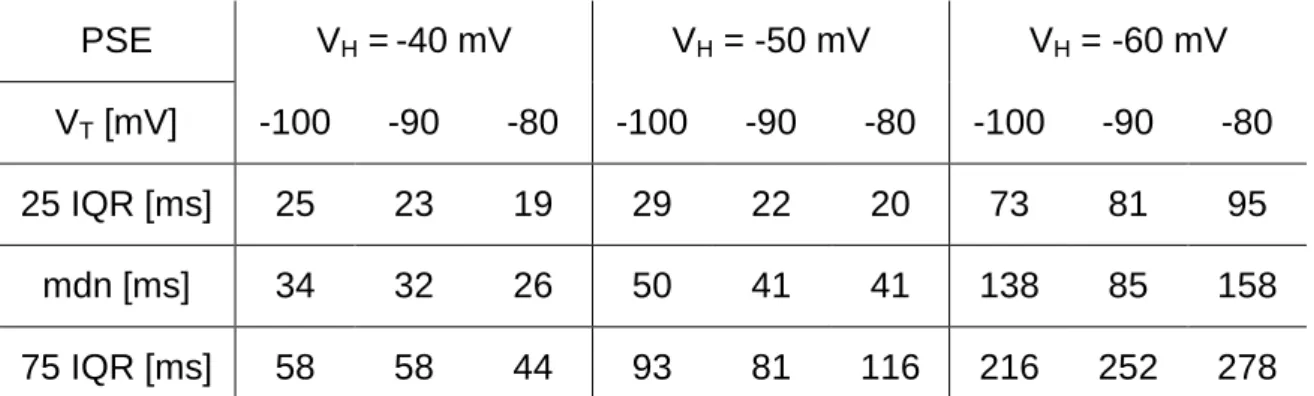
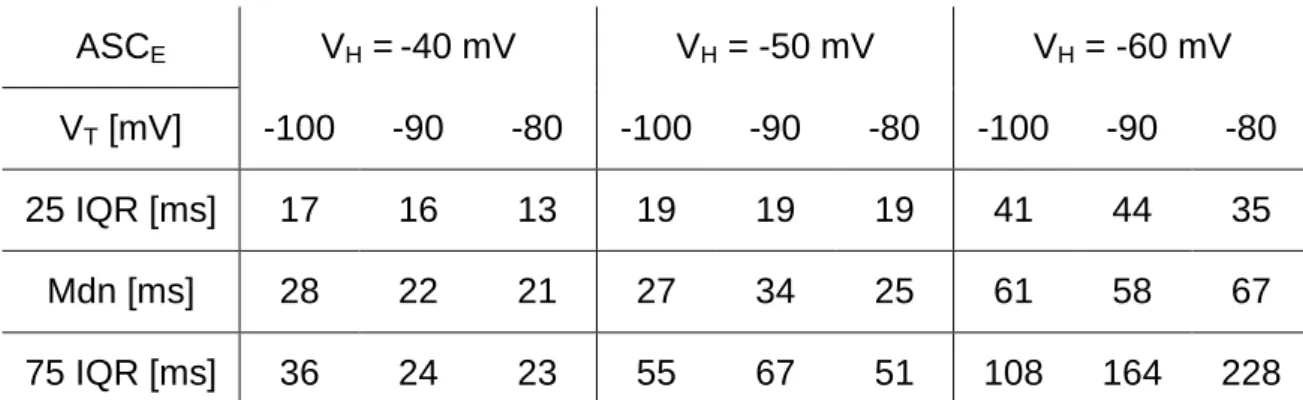
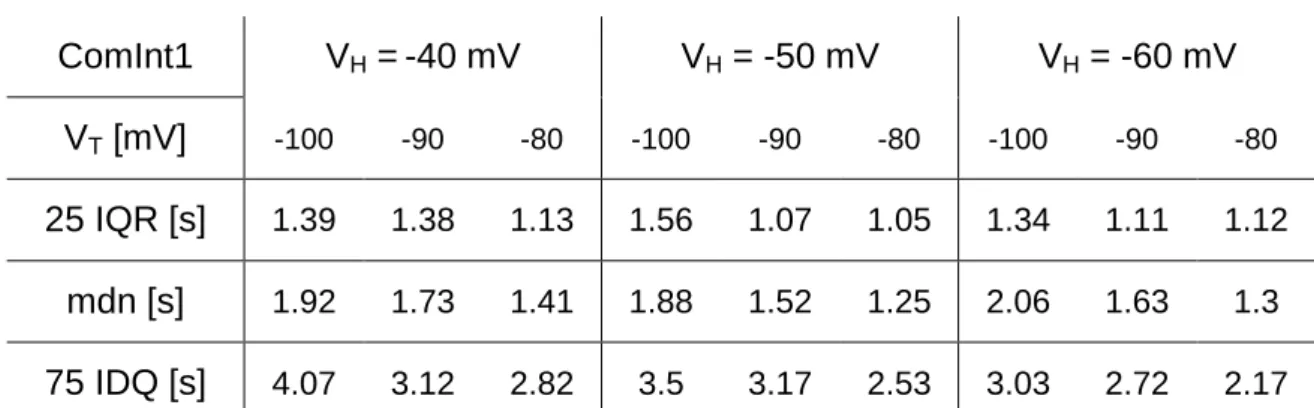
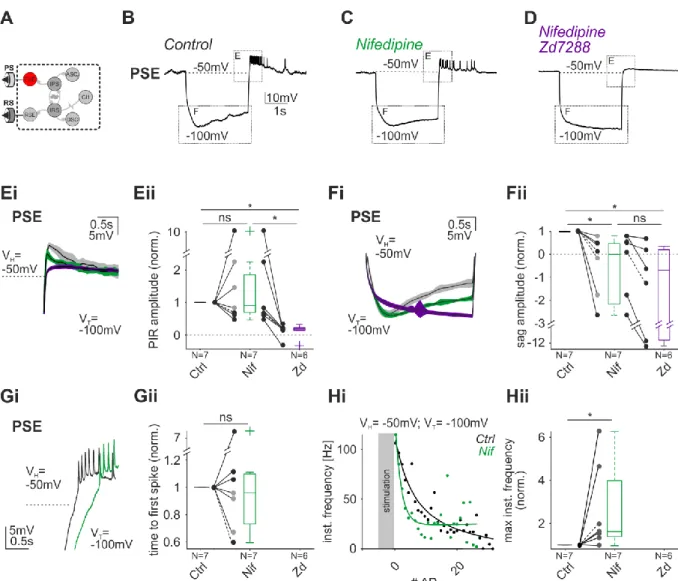
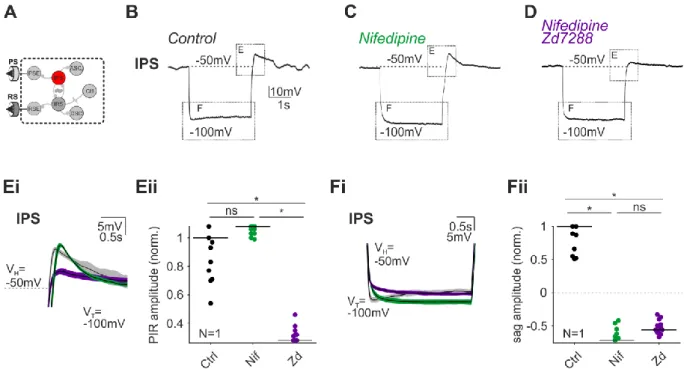
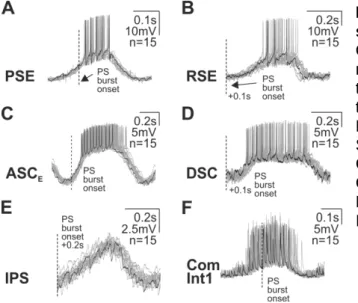
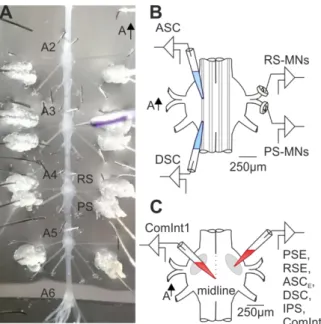
Ionic currents and intrinsic properties of key interneurons and their influence on network activity in a chain of coupled oscillators
Volltext
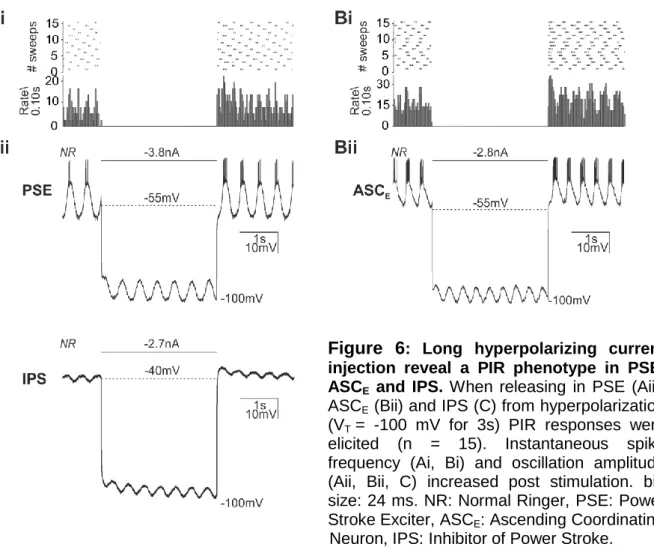
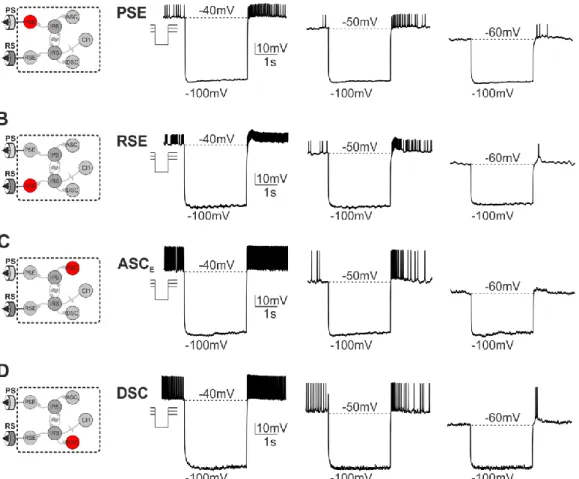
Abbildung
ÄHNLICHE DOKUMENTE
Finally, it is our conjecture that Classic Actors and Processes are mostly used in a fine-grained concurrency setting and lead to a style of programming where the state of an
11 Based on the discussion above, it is obvious that, a higher rewetting temperature means an earlier initiation of the rewetting which will shorten the whole quenching
In Figure 4.12, there is also a noticeable influence displayed due to a change in film thickness, especially in the region, where the transition from a transparent to an opaque
-I- Zone II is characterized by high substrate temperatures (0.3 < T/T m < 0.5) at which both surface and grain boundary diffusion occur at significant levels. The grain
While only considering those oscillating mitochondria that belong to the largest frequency cluster, the findings reveal significant correlations between
Moreover, expression of constitutively active Notch1 in hematopoietic progenitors resulted in a severely altered lymphoid development in the bone marrow, namely a block in
Temperature As the water vapor variation with the 27-day solar forcing is not consistent with the results from the analysis of NLC properties, especially concerning the phase lag of
In this respect Peripheral Bursters are similar to the cardiac ganglion oiPortunus (Benson, 1980), the PD-unit in the stomatogastric ganglion of the lobster (Ayers &