Adipose-derived stem cells in alveolar cleft management:
looking for a better scaffold
Aus Fettgewebe gewonnene Stammzellen im Alveolarspalt-Management:
Suche nach einem besseren Gerüst
Abstract
Background:Although various sources of bone graft material have been suggested in the literature for alveolar cleft reconstruction including
Helmy Soliman
1Quitaibah Al-Kandari
2autogenous, allogenic, xenogenic, and alloplastic grafts, autogenous
Hossam Ismail
3bone graft from either the iliac crest or the tibial plateau remains the
Omar Shouman
3gold standard against which other sources are evaluated. However, the procedure is invasive and associated with a potential risk of early
Ahmed Bahaa El-Din
3complications such as bleeding, pain, infection, fracture or late compli-
Mohammed El Hadidy
3cations such as chronic pain, scarring, paresthesia, and gait abnormal- ities. Moreover, its failure rate is about 15%.
In an effort to improve outcome and decrease donor site morbidity, we tried to find a better scaffold for adipose-derived stem cells (ACSs) as an alternative to autologous bone graft for alveolar cleft reconstruction.
1 Plastic Surgery Department, Mataria Teaching Hospital, GOTHI, Cairo, Egypt Aim of the study:To study the efficacy of demineralized bone matrix
(DBM) versus autologous bone graft as a scaffold for adipose-derived 2 Ibn Sina Hospital, Ministry of Health, Kuwait, Kuwait stem cells (ASCs) and to compare both techniques to the standard
autologous iliac crest bone graft (ICBG). 3 Plastic Surgery Department,
Faculty of Medicine, Methods:54 patients underwent alveolar cleft reconstruction at the
age of mixed dentition over a 3-year period. Their mean age was Mansoura University, Mansoura, Egypt 11.4 years and their mean postoperative follow-up was 12.4 months.
Of these, 18 constituted the ICBG group (standard group), 20 constituted the ACSs with ICBG scaffold (ASCs/ICBG) group, whereas the remaining 16 patients made up ACSs with DBM (ASCs/DBM) group. Results were assessed by rating the radiographs obtained 6 months postoperatively according to Bergland scale.
Results:Alveolar cleft repairs using cancellous bone only (ICBG group) were 72.2 percent successful, alveolar cleft repairs using cancellous bone enhanced with ASCs (ASCs/ICBG group) were 90 percent success- ful, and alveolar cleft repairs using DBM enhanced with ASCs (ASCs/DBM group) were 62.5 percent successful. However, there was no statistical difference between the groups. A significantly shorter op- erative time (average time saving was 103 minutes/case) and higher infection rate (18.8 percent) were observed in ASCs/DBM group as compared to the other two groups.
Conclusion: Although not being significantly better than DBM, ICBG appears to be a good scaffold for ASCs that improves results of alveolar cleft reconstruction.
Keywords:alveolar cleft reconstruction, stem cells, demineralized bone matrix
Zusammenfassung
Hintergrund:Obwohl in der Literatur verschiedene Quellen für Knochen- transplantatmaterial für die Alveolarspaltenrekonstruktion einschließlich autogener, allogener, xenogener und alloplastischer Transplantate vorgeschlagen werden, bleibt autogenes Knochentransplantat aus dem Beckenkamm oder dem Tibiaplateau der Goldstandard, anhand dessen andere Quellen bewertet werden. Das Verfahren ist jedoch invasiv und mit einem möglichen Risiko für frühe Komplikationen wie Blutungen, Schmerzen, Infektionen und Frakturen oder späte Komplikationen wie chronische Schmerzen, Narbenbildung, Parästhesien und Ganganoma- lien verbunden. Darüber hinaus beträgt die Misserfolgsrate etwa 15%.
In dem Bestreben, das Outcome zu verbessern und die Morbidität auf Seiten der Spender zu verringern, haben wir versucht, ein besseres Gerüst für aus Fettgewebe gewonnene Stammzellen als Alternative zu autologem Knochentransplantat für die Alveolarspaltenrekonstruktion zu finden.
Ziel der Studie:Untersuchung der Wirksamkeit der demineralisierten Knochenmatrix gegenüber autologem Knochentransplantat als Gerüst für aus Fettgewebe gewonnene Stammzellen und Vergleich beider Techniken mit dem Standard – dem autologen Beckenkammknochen- transplantat
Methoden:Bei 54 Patienten im Alter des Wechselgebisses wurde über 3 Jahre eine Alveolarspaltenrekonstruktion durchgeführt. Ihr mittleres Alter war 11,4 Jahre und ihr mittleres postoperatives Follow-up betrug 12,4 Monate. Die erste Gruppe umfasste 18 Patienten mit autologen Beckenkammknochentransplantaten (Standard-Gruppe), die zweite Gruppe umfasste 20 Patienten mit aus Fettgewebe gewonnenen Stammzellen mit Beckenkammknochentransplantat, die dritte Gruppe umfasste 16 Patienten mit aus Fettgewebe gewonnenen Stammzellen mit demineralisierter Knochenmatrix. Die Ergebnisse wurden beurteilt, indem die 6 Monate postoperativ gemachten Röntgenaufnahmen nach der Berglandskala bewertet wurden.
Ergebnisse:Alveolarspaltreparaturen nur mit Spongiosa (Beckenkamm- knochentransplantat) waren in 72,2 Prozent erfolgreich. Unter Verwen- dung von Spongiosa, die mit aus Fettgewebe gewonnenen Stammzellen verbessert wurden, waren die Alveolarspaltreparaturen zu 90 Prozent und bei Verwendung von demineralisierter Knochenmatrix, die mit aus Fettgewebe gewonnenen Stammzellen verbessert wurde, zu 62,5 Pro- zent erfolgreich. Allerdings gab es keinen statistisch signifikanten Un- terschied zwischen den Gruppen. Es wurde eine deutlich kürzere Ope- rationszeit (die durchschnittliche Zeitersparnis betrug 103 Minuten/Fall) und eine höhere Infektionsrate von 18,8 Prozent wurden in der Gruppe mit aus Fettgewebe gewonnenen Stammzellen mit entmineralisierter Knochenmatrix im Vergleich zu den anderen zwei Gruppen beobachtet.
Schlussfolgerung:Obwohl Beckenkammknochentransplantat nicht si- gnifikant besser als demineralisierte Knochenmatrix ist, scheint das Beckenkammknochentransplantat ein gutes Gerüst für aus Fettgewebe gewonnene Stammzellen zu sein, wodurch die Ergebnisse der Alveolar- spaltenrekonstruktion verbessert werden.
Schlüsselwörter:Alveolarspaltenrekonstruktion, Stammzellen, demineralisierte Knochenmatrix
Introduction
Repairing the alveolar cleft is an important step to create a stable and continuous maxillary dental arch, facilitate closure of the oronasal fistula, improve support of teeth adjacent to the cleft site, permit further orthodontic and orthognathic interventions, and to provide support to the alar base of the nose.
Although various sources of bone graft material have been suggested in the literature for alveolar cleft recon- struction including autogenous, allogenic, xenogenic, and alloplastic grafts, autologous bone grafts either from the iliac crest or the tibial plateau remain the gold standard against which other graft materials are evaluated [1], [2], [3].
However, the procedure is invasive and associated with a potential risk of early complications such as bleeding, pain, infection, fracture, and/or late complications such as chronic pain, scarring, paresthesia, and gait abnormal- ities [4], [5]. Moreover, its failure rate is about 15% [6], [7].
For effective osteogenesis to take place, presence of both osteoinductive factors and osteoconductive scaffolds are needed. Cancellous bone grafts, either from iliac crest or tibial plateau display both osteoinductive and osteocon- ductive properties, which explain their efficacy in a wide variety of procedures.
Demineralized bone matrix (DBM) is an established group of allograft bone substitutes that has been used extens- ively in the orthopedic surgery as an osteoconductive scaffold but it has no osteoinductive potentiality.
ACSs act not only through direct bone formation in the gap of alveolar cleft, but also due to their paracrine ef- fects: production of extracellular matrix, releasing cytokines and promotion of angiogenesis. ACSs in com- bination with a proper scaffold have a great potential that has already been proven in animal studies and on hu- mans [8], [9], [10], [11], [12], [13], [14], [15], [16], [17], [18], [19].
Our aim is to study the efficacy of demineralized bone matrix versus autologous bone graft as a scaffold for adipose derived stem cells (ASCs) and to compare both techniques to the standard autologous ICBG for alveolar cleft reconstruction.
Patients and methods
After getting the approval from the Ethical Committee of Faculty of Medicine, Mansoura University (Study No.
CD60), fifty-four patients with unilateral alveolar cleft were collected randomly from the outpatient clinics of the Plastic Surgery Centre at Mansoura University and El Mataria Teaching Hospital over a 3-year period from March of 2012 to February of 2015. All patients with unilateral or bilateral alveolar cleft were included, and patients operated before and needed revision (due to previous failure or inadequate bone formation) were also included. Old patients (>12 years) with neglected alveolar
cleft with no aesthetic or functional concerns (e.g. no alar depression, no need for orthognathic surgery or dental implants), patients with small alveolar notch only, and patient with mental disorders were excluded. Their mean age was 11.4 years and their mean postoperative follow- up was 12.4 months.
For each patient, age, sex, medical and surgical history was recorded.
All patients were requested to have preoperative and 6 months postoperative periapical, occlusal, and panor- amic radiograph.
All patients and/or their parents were offered the three modalities of treatment. In addition to standard ICBG, they are offered the possibility of an alternative procedure using ACSs on an off-label basis, along with autogenous ICBG or DBM as a scaffold, and the choice of a specific modality was according to each patient’s (or his/her parents’) preference.
Patients were then divided into three groups according to the material(s) used for grafting. Of the 54 patients, 18 constituted the ICBG group (standard group), 20 constituted the ACSs with ICBG scaffold (ASCs/ICBG) group, whereas the remaining 16 patients made up ACSs with DBM (ASCs/DBM) group.
Operative technique
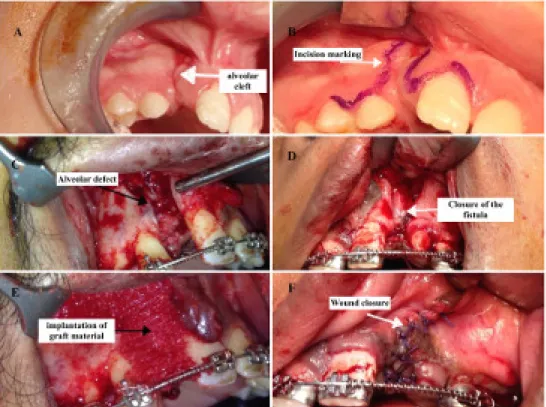
The standard Gingivoperiosteoplasty technique was per- formed as described by Skoog [20] to prepare the appro- priate pocket for introducing our graft (Figure 1).
Lidocaine 1% with epinephrine 1:200,000 was infiltrated around the cleft margins, then intraoperative reassess- ment of the cleft was done as regard its extent, position of the teeth on the margins of the cleft, and presence of nasoalveolar fistula. An incision was made around the labial component of the fistula, first within the loose mu- cosa, then within the alveolar processes. The incision was continued along the margin of the alveolar cleft ver- tically toward the crest of the alveolus on each side, posi- tioned equidistant between the labial and palatal sur- faces. Once on the alveolar crest, the incisions were car- ried within the gingival sulci of the teeth on their labial aspect. Within the lesser segment, the incision was typic- ally extended to the second premolar. Next, the mucoperi- osteum was dissected from the alveolar processes on the labial aspect using periosteal elevator. This dissection extended to the nasal floor, exposing the lateral aspect of the anterior nasal spine and the lower pyriform rim.
Through the labial approach, the mucoperiosteum was elevated off the bony walls of the cleft from the alveolar crest to the nasal floor. The oronasal fistula tract was then dissected and closed by sutures. At this time, before placement of the bone graft, adequate labial soft tissue mobility that will provide a tension-free closure over the bone graft was confirmed. If greater mobility was needed, horizontal scoring of the periosteum at the base of the lesser segment flap was done. Extending the back cut and directing it anteriorly gained further mobility if needed. After introducing the graft material(s), the lesser
Figure 1: Surgical technique. A: alveolar cleft, B: incision marking, C: elevation of gingivoperiosteal flaps and exposing the defect, D: closure of oronasal fistula, E: implantation of graft material, F: wound closure
segment mucoperiosteal flap was advanced medially and slightly palatally to cover the bone graft and to provide the oral labial closure of the fistula using interrupted 4/0 Vicryl (Ethicon, Inc., Somerville, NJ) sutures. Interrupted interdental papilla sutures were then placed to stabilize the labial and palatal tissues toward one another and against the bony alveolar process.
Harvesting cancellous bone from the iliac crest
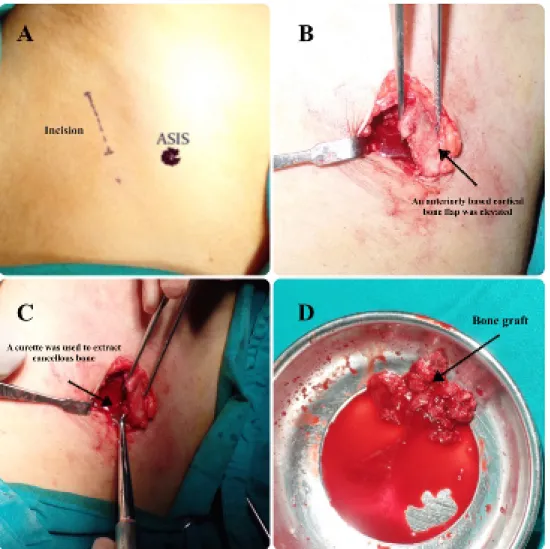
The cancellous bone is harvested from the iliac crest using the standard technique, taking into consideration not to place the scar on the bone prominence (1 cm posterior and lateral to anterior superior iliac spine (ASIS), Figure 2).
3 cc of the tumescent solution was injected at the incision site; incision was then taken by 15 blade through the skin and continued by diathermy down to the periosteum. In- cision was then taken through the periosteum to expose the bone, and an osteotome was used to make a trap door fenestration (two vertical cuts and one from the medial aspect of the crest to connect the vertical cuts), then an anteriorly based cortical bone flap was elevated to expose the cancellous bone. A curette was then used to extract as much cancellous bone as needed. The cor- tical roof was then reduced back in place to cover the donor site and reduce the postoperative bleeding. If there were any defects or fragmentation of the cortex of the roof, bone wax was used to seal the cancellous bone cavity. Vicryl 3/0 (Ethicon, Inc., Somerville, NJ) sutures were used for the re-attachment of the muscles and for
closure of the subcutaneous layer, and Monocryl 4/0 (Ethicon, Inc., Somerville, NJ) sutures were used in a subQ pattern to close the skin. 1 cc of Xylocaine 1% +1 cc of Marcaine 0.25% was injected to relief postoperative pain and soft dressing was applied.
Preparation of the demineralized bone matrix
Using sterile technique on a side table with gloves, the demineralized bone matrix powder (Wright Medical Technology, Inc., Arlington, TN) was emptied into the mixing bowl (5 cc).
The mixing solution was then emptied into the bowl gradually and mixed with spatula and the material kneaded against the sidewall of the bowl until the desired consistency was achieved (approximately 30–60 seconds).
After achieving a putty-like consistency, the material can be handled digitally. Material maintains handling charac- teristics up to 10 minutes after mixing, during that period it was implanted in the already prepared cleft area.
Preparation of adipose-derived stem cells from the fat
Fat grafts were harvested at the end of the procedure.
Donor site was the lower abdomen in all cases. In ACSs/ICBG group, the aspiration cannula was introduced through the same incision of bone graft harvesting to avoid additional scarring. In ACSs/DBM group, the aspir- ation cannula was introduced through a small 0.5 cm
Figure 2: Harvesting cancellous bone from iliac crest. A: incision is marked 1–2 cm posterior and laterally to ASIS, B: an anteriorly based cortical bone flap was elevated, C: A curette was used to extract cancellous bone, D: harvested cancellous bone graft
stab incision in the umbilicus at 6 o’clock. Liposuction was performed as described by SR Coleman [21]. A long a traumatic 3 mm Mercedes cannula (luer lock type) was used. An average amount of 60 cc was usually harvested (range 50–80 cc). The raw aspirate was then collected for stem cell preparation.
Fat was extensively washed with sterile phosphate buf- fered saline (PBS) to remove the blood cells, saline, and local anesthetics. Then ACSs were separated from adipose tissue by cell digestion using 0.075% collagenase type I solution (Collagenase NB4 Standard, SERVA Electrophoresis, Heidelberg, Germany) at 37°C for 30 min to one hour.
An equal volume of Dulbecco’s Modified Eagle Medium (DMEM) containing 10% fetal bovine serum (FBS) and 1% penicillin–streptomycin (Gibco, Carlsbad, CA, USA) was then added to inactivate collagenase. The digest was centrifuged at 1,500 rpm for 5 min. The supernatant was removed, and the cell pellet, termed the stromal vascular fraction (SVF), was left.
The SVF, containing ASCs, was resuspended in 10% FBS then recentrifuged and filtered through a 100-lm nylon filter. The cell pellet was re-suspended in a 10 ml com- plete culture medium formed of DMEM, 13% FBS and 1.5% penicillin streptomycin mixture (Lonza, Verviers,
Belgium). The cell suspension was cultured in culture flask 25 cm2(Easy Flask, Nunc, Roskilde, Denmark) and incubated in CO2incubator (Nuaire, NU 4950E, Autoflow Water Jacketed CO2incubator, USA) at 37°C and 5% CO2. The ASCs were prepared in a final concentration of 3x106/ml and its identity was confirmed by flow cytometry (positive for CD49, CD71, CD73, CD90, CD105, and negative for CD31, CD34, CD45), and then supplied in a tube for injection.
Postoperative follow-up
All patients instructed to have clear fluids only for the first 48 hours after surgery, then soft diet for 4 weeks. Antibi- otics prophylaxis was given before surgery, and for 3 days after surgery. Mouthwash was used regularly after meals for one week. All patients were stable and discharged from the hospital in the second postoperative day.
Follow-up visit was scheduled in the outpatient clinic one week after surgery when checking of the oral wound and dressing of the graft doner site (in ICBG and ASCs/ICBG groups) were done.
Prepared ASCs was injected in the pocket of the bone graft at that time using a 27-gauge needle (for ASCs/ICBG
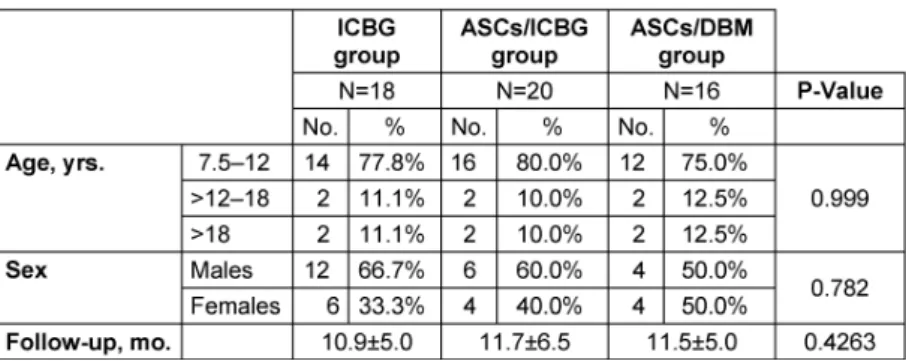
Table 1: Characteristics of the groups by type of repair
Table 2: Comparison between groups according to success of bone grafts
and ASCs/DBM groups). By that time, the mucosa was already healed and the pocket is sealed.
Radiographic outcomes
In this study, the success of autogenous bone grafts was assessed through evaluating the radiographs taken at 6 months postoperatively using the indicators of surgical success described by Bergland (Figure 3) [22]. Bone grafts of types I and II, according to the Bergland scale, were considered successful bone grafts, whereas the other types were considered unsuccessful.
Figure 3: Bergland scale: The amount of bone produced in the cleft site was evaluated based on the height of the intraalveolar
septum (bone bridge).
Heights are measured from the apical extent of the cleft site (a line between the tips of the roots of the adjacent teeth) to the cementoenamel junction coronally. Type 1 is defined as normal height (more than 75% of the normal bone height). Type 2 is defined as less than normal height (50–75% of normal bone height). Type 3 has less than 50% of normal bone height. Type
4 has no bone bridge in the cleft site.
Statistical analysis
GraphPad Prism version 5 for Mac (GraphPad Software, Inc., La Jolla, Calif.) was used to carry out statistical tests.
The Fisher exact test was used to analyze the data com- paring the success rate, the complications rate and the operative time between the ASCs/ICBG group, the ASCs/DBM group and the standard ICBG group. P-value was regarded as significant if less than 0.05.
Results
All groups were similar as regard number of patients, age, sex, follow-up period, and time between alveolar recon- struction and postoperative radiographs (Table 1).
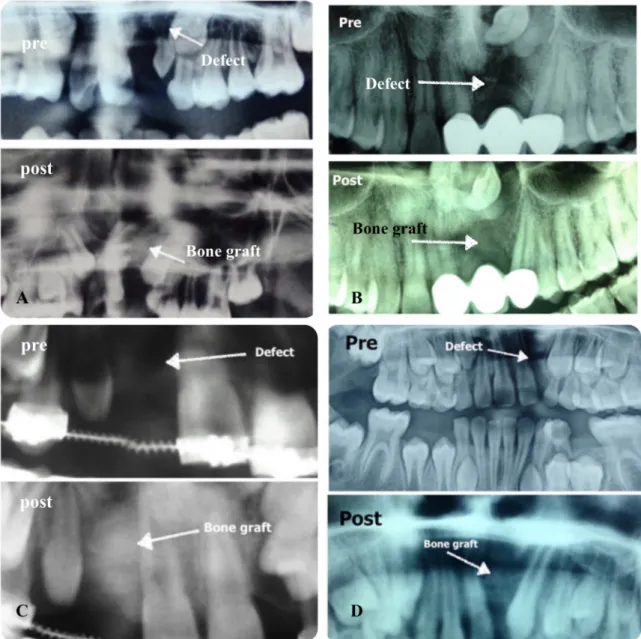
Alveolar cleft repairs using cancellous bone only (ICBG group) were 72.2 percent successful, alveolar cleft repairs using cancellous bone with ASCs (ASCs/ICBG group) were 90 percent successful, and alveolar cleft repairs using DBM with ASCs (ASCs/DBM group) were 62.5 percent successful. However, there were no statistical difference between the groups (P-value >0.05) (Table 2, Figure 4, Figure 5).
Figure 5: Pre- and postoperative radiographs of some cases. A: ICBG was used, B and C: ASCs/ICBG was used, D: ASCs/DBM was used.
Figure 4: Comparison between groups according to success of bone grafts.
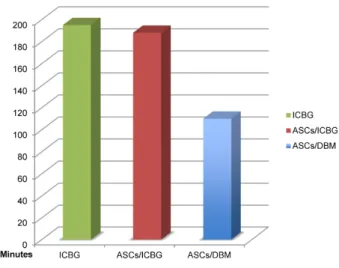
Operative time in ASCs/DBM group was significantly shorter than in the other two groups (P-value <0.001), the average time saving was 103 minutes/case (Table 3, Figure 6).
A significantly higher cleft site infection rate was observed in the ASCs/DBM group (18.8 percent) as compared to ICBG group (standard group). The oronasal fistula was completely closed in all patients except two, one in each of ICBG and ASCS/DBM groups.
Table 3: Mean operative time
Figure 6: Mean operative time
Discussion
Despite the superiority of ICBG, and despite being the
‘‘gold standard bone graft’’ for reconstruction of alveolar cleft and the standardized graft to which different types of bone grafts are compared [23], two major disadvan- tages remain. First, its associated donor-site morbidities including delayed ambulation, pain, hematoma, discom- fort, nerve injury, prolonged hospitalization, and limited volume that can be harvested. Moreover, its failure rate is about 15% [24].
So, in an effort to reduce the donor-site morbidity, many other sources of bone graft material have been suggested in the literature for alveolar cleft reconstruction including allogenic, xenogenic, and alloplastic grafts. The outcomes achieved with different graft materials have been exten- sively studied and compared in the literature, and each type has its advantages and disadvantages. But the ideal bone graft material remains controversial till now [25].
In the present study, fifty-four patients were chosen ran- domly and divided into three groups according to the graft material(s) used: ICBG group (the control group), in which we used cancellous bone only; ASCs/ICBG group, in which ACSs with ICBG scaffold was used; and ASCs/DBM group, in which we used ASCs with DBM scaffold. To the best of our knowledge, this is the first prospective study that used the ICBG and/or DBM scaffold with adipose-derived stem cells for alveolar cleft reconstruction till the time of launching our study, and this is the unique part of our research.
In this study we chose adipose tissue as a source for the stem cells similar to Gimble et al. [26], Zuk et al. [27], and Yoshimura et al. [28]. Others, like Behnia et al. [16], Hibi et al. [14], and Pradel et al. [15] used mesenchymal stem cells from the bone marrow. The advantage of adipose tissue is that it represents an abundant, reliable, noninvasive, and accessible source of stem cells.
Lipoaspirate provides an easily accessible source of ASCs at a frequency of 1:100 to 1:1,500 cells. With 1 g of adipose tissue contains nearly 5,000 ASCs. This greatly exceeds the frequency of other multipotent cells such as mesenchymal stem cells (MSCs) from bone marrow 500-
fold [29], [30]. We confirmed that ASCs express charac- teristic surface markers by flow cytometry (positive for CD49, CD71, CD73, CD90, CD105, and negative for CD31, CD34, CD45), according to the criteria laid by the IFATS & ISCTin 2013.
It has been proven in the literatures that ACSs have the capability to differentiate into various lineages including osteogenic [26], [27]. Guasti et al. examined ACSs derived from abdominal adipose tissue in pediatric population and confirmed their proliferation and differentiation properties with an ability of selective osteogenic [31].
In preclinical studies ACSs were found compatible with different scaffolds including collagen, titanium dioxide, and tricalcium phosphate [32], [33], [34]. ACSs were also successfully used in combination with fibrin glue to cover a large calvarial traumatic defect in a 7-year-old patient [35], but no studies used ACSs with a DBM scaffold.
Hibi et al. published the first clinical use of stem cells for bone tissue engineering in a 9-year-old patient with 10x13 mm alveolar cleft. Mesenchymal stem cells (MSCs) were obtained from bone marrow, cultured for 4 weeks, and then differentiated into osteogenic lineage. He used a ti- tanium-mesh plate over which MSC, platelets rich plasma (PRP), and calcium chloride solution with thrombin were applied using a syringe. Nine months later, 79.1% of the bone was regenerated, and the canine and the lateral incisor erupted forcing out the mesh plate [14].
Pradel et al. reported spontaneous tooth eruption and complete defect closure after filling the alveolar cleft de- fect with MSCs harvested from the maxilla in a bovine collagen matrix scaffold in a 10-year-old male [15].
Behnia et al. utilized mesenchymal stem cells from the bone marrow carried on a scaffold that combined demin- eralized bone and calcium sulfate for alveolar cleft recon- struction. The results suggested that the amount of bone formation was inadequate and indicated that the conven- tional bone substitute was a favorable scaffold for mes- enchymal stem cells for alveolar bone regeneration [16].
In the present study, we used demineralized bone matrix (DBM) as an osteoconductive scaffold for alveolar cleft reconstruction in ASCs/DBM group patients similar to Cameron et al. [36], Behnia et al. [16], Sivak et al. [37], Macisaac et al. [38], and Louis et al. [39]. And we used the cancellous bone harvested from the iliac crest as a scaffold for ACSs in ASCs/ICBG group patients similar to Kom et al. [40], Behnia et al. [16], and Yuanzheng et al.
[41], 2015 who enhanced the autologous iliac bone with MSCs from the iliac crest.
Others, like Benliday et al. [42] used bovine hydroxia- petite, Pradel et al. [15] used bovine collagen matrix, De Ruiter et al. used tri-calcium phosphate [43]. Jiao et al.
used cryopreserved dentin matrix as a scaffold [44].
DBM has the advantages of being osteoconductive. It does cause local foreign-body immunogenic reaction as the antigenic surface structure of the bone is destroyed during demineralization, its degradation does not produce any products that affect new bone formation, and being prepared by acid extraction of allografts, it retains colla- gen and other proteins as well as bone morphogenetic
proteins (BMPs) which have osteoinductive capability.
Although, transmission of diseases has not yet been re- ported with DBM but is theoretically possible [45].
Many factors have been used in the literatures to enhance either cancellous bone or DBM to improve bone forma- tion. The most frequently used are PRP and BMPs [46], [47].
Backly et al. demonstrated that PRP enhances late stage bone regeneration with osteoinductive effects that last for 8 weeks [48]. Dutra et al. demonstrated that PRP improves bone formation in artificially induced alveolar defects when combined with bioactive glass foams [49].
But, Luaces et al. found no differences between control and study groups who received PRP and autologous bone for alveolar cleft reconstruction [50].
Cameron et al. compared the results of using DBM en- hanced with BMPs with the conventional cancellous bone from the iliac crest and they found better results in the group that used DBM with BMPs with success rate of 97.2% compared to 84.2% for the group used cancellous bone from the iliac crest, with significant decrease in the operative time [36]. Canan et al. [51] and Fallucco et al.
[52] used recombinant human BMP in treating patients with alveolar clefts and the results were comparable to autologous ICBG. Others, as Neovius et al. had to termin- ate the study because of severe gingival swelling in pa- tients receiving effective doses of BMP-2 [53].
In this study, a statistically non-significant difference was detected in the success rates between the three groups.
However, we found a significant higher infection rate and significant shorter operative time in ASCs/DBM group as compared to the other control group, the average time saving was 103 minutes/case. This is similar to Cameron et al. who found significant shorter operative time when DBM was used [38].
The limitation of our study was the small number of the patient in each group that might be the reason for non- significant results.
Notes
Competing interests
The authors declare that they have no competing interests.
References
1. Cohen M, Figueroa AA, Haviv Y, Schafer ME, Aduss H. Iliac versus cranial bone for secondary grafting of residual alveolar clefts.
Plast Reconstr Surg. 1991 Mar;87(3):423-7; discussion 428.
DOI: 10.1097/00006534-199103000-00004
2. Jia YL, Fu MK, Ma L. Long-term outcome of secondary alveolar bone grafting in patients with various types of cleft. Br J Oral Maxillofac Surg. 2006 Aug;44(4):308-12. DOI:
10.1016/j.bjoms.2005.07.003
3. Horswell BB, Henderson JM. Secondary osteoplasty of the alveolar cleft defect. J Oral Maxillofac Surg. 2003
Sep;61(9):1082-90. DOI: 10.1016/S0278-2391(03)00322-7 4. Gimbel M, Ashley RK, Sisodia M, Gabbay JS, Wasson KL, Heller
J, Wilson L, Kawamoto HK, Bradley JP. Repair of alveolar cleft defects: reduced morbidity with bone marrow stem cells in a resorbable matrix. J Craniofac Surg. 2007 Jul;18(4):895-901.
DOI: 10.1097/scs.0b013e3180a771af
5. Moreau JL, Caccamese JF, Coletti DP, Sauk JJ, Fisher JP. Tissue engineering solutions for cleft palates. J Oral Maxillofac Surg.
2007 Dec;65(12):2503-11. DOI: 10.1016/j.joms.2007.06.648 6. Paganelli C, Fontana P, Porta F, Majorana A, Pazzaglia UE, Sapelli
PL. Indications on suitable scaffold as carrier of stem cells in the alveoloplasty of cleft palate. J Oral Rehabil. 2006 Aug;33(8):625- 9. DOI: 10.1111/j.1365-2842.2005.01594.x
7. Schultze-Mosgau S, Nkenke E, Schlegel AK, Hirschfelder U, Wiltfang J. Analysis of bone resorption after secondary alveolar cleft bone grafts before and after canine eruption in connection with orthodontic gap closure or prosthodontic treatment. J Oral Maxillofac Surg. 2003 Nov;61(11):1245-8. DOI: 10.1016/S0278- 2391(03)00722-5
8. Yoshioka M, Tanimoto K, Tanne Y, Sumi K, Awada T, Oki N, Sugiyama M, Kato Y, Tanne K. Bone regeneration in artificial jaw cleft by use of carbonated hydroxyapatite particles and mesenchymal stem cells derived from iliac bone. Int J Dent.
2012;2012:352510. DOI: 10.1155/2012/352510
9. Tanimoto K, Sumi K, Yoshioka M, Oki N, Tanne Y, Awada T, Kato Y, Sugiyama M, Tanne K. Experimental Tooth Movement Into New Bone Area Regenerated by Use of Bone Marrow-Derived Mesenchymal Stem Cells. Cleft Palate Craniofac J. 2015 07;52(4):386-94. DOI: 10.1597/12-232
10. Zhang D, Chu F, Yang Y, Xia L, Zeng D, Uludağ H, Zhang X, Qian Y, Jiang X. Orthodontic tooth movement in alveolar cleft repaired with a tissue engineering bone: an experimental study in dogs.
Tissue Eng Part A. 2011 May;17(9-10):1313-25. DOI:
10.1089/ten.TEA.2010.0490
11. Ou XR, Jian XC, Lin G. [An investigation of restoration of alveolar cleft with engineered bone]. Zhonghua Zheng Xing Wai Ke Za Zhi. 2007 Jan;23(1):29-31.
12. Pourebrahim N, Hashemibeni B, Shahnaseri S, Torabinia N, Mousavi B, Adibi S, Heidari F, Alavi MJ. A comparison of tissue- engineered bone from adipose-derived stem cell with autogenous bone repair in maxillary alveolar cleft model in dogs. Int J Oral Maxillofac Surg. 2013 May;42(5):562-8. DOI:
10.1016/j.ijom.2012.10.012
13. Korn P, Schulz MC, Range U, Lauer G, Pradel W. Efficacy of tissue engineered bone grafts containing mesenchymal stromal cells for cleft alveolar osteoplasty in a rat model. J Craniomaxillofac Surg. 2014 Oct;42(7):1277-85. DOI:
10.1016/j.jcms.2014.03.010
14. Hibi H, Yamada Y, Ueda M, Endo Y. Alveolar cleft osteoplasty using tissue-engineered osteogenic material. Int J Oral Maxillofac Surg. 2006 Jun;35(6):551-5. DOI: 10.1016/j.ijom.2005.12.007 15. Pradel W, Tausche E, Gollogly J, Lauer G. Spontaneous tooth
eruption after alveolar cleft osteoplasty using tissue-engineered bone: a case report. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2008 Apr;105(4):440-4. DOI:
10.1016/j.tripleo.2007.07.042
16. Behnia H, Khojasteh A, Soleimani M, Tehranchi A, Khoshzaban A, Keshel SH, Atashi R. Secondary repair of alveolar clefts using human mesenchymal stem cells. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2009 Aug;108(2):e1-6. DOI:
10.1016/j.tripleo.2009.03.040
17. Behnia H, Khojasteh A, Soleimani M, Tehranchi A, Atashi A. Repair of alveolar cleft defect with mesenchymal stem cells and platelet derived growth factors: a preliminary report. J Craniomaxillofac Surg. 2012 Jan;40(1):2-7. DOI: 10.1016/j.jcms.2011.02.003 18. Stanko P, Mracna J, Stebel A, Usakova V, Smrekova M, Vojtassak
J. Mesenchymal stem cells - a promising perspective in the orofacial cleft surgery. Bratisl Lek Listy. 2013;114(2):50-2. DOI:
10.4149/BLL_2013_012
19. Chai G, Zhang Y, Hu XJ, Wang M, Liu W, Cui L, Cao YL. [Repair alveolar cleft bone defects with bone marrow stromal cells].
Zhonghua Zheng Xing Wai Ke Za Zhi. 2006 Nov;22(6):409-11.
20. Skoog T. The use of periosteum and Surgicel for bone restoration in congenital clefts of the maxilla. A clinical report and experimental investigation. Scand J Plast Reconstr Surg.
1967;1(2):113-30.
21. Coleman SR. Structural fat grafts: the ideal filler? Clin Plast Surg.
2001 Jan;28(1):111-9.
22. Bergland O, Semb G, Abyholm F, Borchgrevink H, Eskeland G.
Secondary bone grafting and orthodontic treatment in patients with bilateral complete clefts of the lip and palate. Ann Plast Surg. 1986 Dec;17(6):460-74. DOI: 10.1097/00000637- 198612000-00005
23. Rawashdeh MA, Telfah H. Secondary alveolar bone grafting: the dilemma of donor site selection and morbidity. Br J Oral Maxillofac Surg. 2008 Dec;46(8):665-70. DOI:
10.1016/j.bjoms.2008.07.184
24. Schultze-Mosgau S, Nkenke E, Schlegel AK, Hirschfelder U, Wiltfang J. Analysis of bone resorption after secondary alveolar cleft bone grafts before and after canine eruption in connection with orthodontic gap closure or prosthodontic treatment. J Oral Maxillofac Surg. 2003 Nov;61(11):1245-8. DOI: 10.1016/S0278- 2391(03)00722-5
25. Ochs MW. Alveolar cleft bone grafting (Part II): Secondary bone grafting. J Oral Maxillofac Surg. 1996 Jan;54(1):83-8. DOI:
10.1016/S0278-2391(96)90311-0
26. Gimble JM, Katz AJ, Bunnell BA. Adipose-derived stem cells for regenerative medicine. Circ Res. 2007 May;100(9):1249-60.
DOI: 10.1161/01.RES.0000265074.83288.09
27. Zuk PA, Zhu M, Mizuno H, Huang J, Futrell JW, Katz AJ, Benhaim P, Lorenz HP, Hedrick MH. Multilineage cells from human adipose tissue: implications for cell-based therapies. Tissue Eng. 2001 Apr;7(2):211-28. DOI: 10.1089/107632701300062859 28. Yoshimura K, Sato K, Aoi N, Kurita M, Hirohi T, Harii K. Cell-
assisted lipotransfer for cosmetic breast augmentation:
supportive use of adipose-derived stem/stromal cells. Aesthetic Plast Surg. 2008 Jan;32(1):48-55; discussion 56-7. DOI:
10.1007/s00266-007-9019-4
29. Rubio D, Garcia-Castro J, Martín MC, de la Fuente R, Cigudosa JC, Lloyd AC, Bernad A. Spontaneous human adult stem cell transformation. Cancer Res. 2005 Apr;65(8):3035-9. DOI:
10.1158/0008-5472.CAN-04-4194
30. Peng L, Jia Z, Yin X, Zhang X, Liu Y, Chen P, Ma K, Zhou C.
Comparative analysis of mesenchymal stem cells from bone marrow, cartilage, and adipose tissue. Stem Cells Dev. 2008 Aug;17(4):761-73. DOI: 10.1089/scd.2007.0217
31. Guasti L, Prasongchean W, Kleftouris G, Mukherjee S, Thrasher AJ, Bulstrode NW, Ferretti P. High plasticity of pediatric adipose tissue-derived stem cells: too much for selective skeletogenic differentiation? Stem Cells Transl Med. 2012 May;1(5):384-95.
DOI: 10.5966/sctm.2012-0009
32. Yang P, Huang X, Wang C, Dang X, Wang K. Repair of bone defects using a new biomimetic construction fabricated by adipose-derived stem cells, collagen I, and porous beta-tricalcium phosphate scaffolds. Exp Biol Med (Maywood). 2013
Dec;238(12):1331-43. DOI: 10.1177/1535370213505827
33. Daei-Farshbaf N, Ardeshirylajimi A, Seyedjafari E, Piryaei A, Fadaei Fathabady F, Hedayati M, Salehi M, Soleimani M, Nazarian H, Moradi SL, Norouzian M. Bioceramic-collagen scaffolds loaded with human adipose-tissue derived stem cells for bone tissue engineering. Mol Biol Rep. 2014 Feb;41(2):741-9. DOI:
10.1007/s11033-013-2913-8
34. Benazzo F, Botta L, Scaffino MF, Caliogna L, Marullo M, Fusi S, Gastaldi G. Trabecular titanium can induce in vitro osteogenic differentiation of human adipose derived stem cells without osteogenic factors. J Biomed Mater Res A. 2014 Jul;102(7):2061- 71. DOI: 10.1002/jbm.a.34875
35. Lendeckel S, Jödicke A, Christophis P, Heidinger K, Wolff J, Fraser JK, Hedrick MH, Berthold L, Howaldt HP. Autologous stem cells (adipose) and fibrin glue used to treat widespread traumatic calvarial defects: case report. J Craniomaxillofac Surg. 2004 Dec;32(6):370-3. DOI: 10.1016/j.jcms.2004.06.002 36. Francis CS, Mobin SS, Lypka MA, Rommer E, Yen S, Urata MM,
Hammoudeh JA. rhBMP-2 with a demineralized bone matrix scaffold versus autologous iliac crest bone graft for alveolar cleft reconstruction. Plast Reconstr Surg. 2013 May;131(5):1107-15.
DOI: 10.1097/PRS.0b013e3182865dfb
37. Sivak WN, Macisaac ZM, Rottgers SA, Losee JE, Kumar AR.
Management of failed alveolar bone grafts: improved outcomes and decreased morbidity with allograft alone. Plast Reconstr Surg. 2014 Feb;133(2):345-54. DOI:
10.1097/01.prs.0000436855.17280.c4
38. Macisaac ZM, Rottgers SA, Davit AJ 3rd, Ford M, Losee JE, Kumar AR. Alveolar reconstruction in cleft patients: decreased morbidity and improved outcomes with supplemental demineralized bone matrix and cancellous allograft. Plast Reconstr Surg. 2012 Sep;130(3):625-32. DOI: 10.1097/PRS.0b013e31825dcb75 39. Louis-Ugbo J, Murakami H, Kim HS, Minamide A, Boden SD.
Evidence of osteoinduction by Grafton demineralized bone matrix in nonhuman primate spinal fusion. Spine. 2004 Feb;29(4):360- 6; discussion Z1. DOI: 10.1097/01.BRS.0000090823.12652.F9 40. Korn P, Schulz MC, Range U, Lauer G, Pradel W. Efficacy of tissue engineered bone grafts containing mesenchymal stromal cells for cleft alveolar osteoplasty in a rat model. J Craniomaxillofac Surg. 2014 Oct;42(7):1277-85. DOI:
10.1016/j.jcms.2014.03.010
41. Yuanzheng C, Yan G, Ting L, Yanjie F, Peng W, Nan B.
Enhancement of the repair of dog alveolar cleft by an autologous iliac bone, bone marrow-derived mesenchymal stem cell, and platelet-rich fibrin mixture. Plast Reconstr Surg. 2015 May;135(5):1405-12. DOI: 10.1097/PRS.0000000000001166 42. Benlidayi ME, Tatli U, Kurkcu M, Uzel A, Oztunc H. Comparison
of bovine-derived hydroxyapatite and autogenous bone for secondary alveolar bone grafting in patients with alveolar clefts.
J Oral Maxillofac Surg. 2012 Jan;70(1):e95-e102. DOI:
10.1016/j.joms.2011.08.041
43. de Ruiter A, Janssen N, van Es R, Frank M, Meijer G, Koole R, Rosenberg T. Micro-structured Beta-Tricalcium Phosphate for Repair of the Alveolar Cleft in Cleft Lip and Palate Patients: A Pilot Study. Cleft Palate Craniofac J. 2015 05;52(3):336-40. DOI:
10.1597/13-260
44. Jiao L, Xie L, Yang B, Yu M, Jiang Z, Feng L, Guo W, Tian W.
Cryopreserved dentin matrix as a scaffold material for dentin- pulp tissue regeneration. Biomaterials. 2014 Jun;35(18):4929- 39. DOI: 10.1016/j.biomaterials.2014.03.016
45. Tuli SM, Singh AD. The osteoninductive property of decalcified bone matrix. An experimental study,. J Bone Joint Surg Br. 1978 Feb;60(1):116-23. DOI: 10.1302/0301-620X.60B1.342532 46. Marukawa E, Oshina H, Iino G, Morita K, Omura K. Reduction of
bone resorption by the application of platelet-rich plasma (PRP) in bone grafting of the alveolar cleft. J Craniomaxillofac Surg.
2011 Jun;39(4):278-83. DOI: 10.1016/j.jcms.2010.04.017
47. Alonso N, Tanikawa DY, Freitas Rda S, Canan L Jr, Ozawa TO, Rocha DL. Evaluation of maxillary alveolar reconstruction using a resorbable collagen sponge with recombinant human bone morphogenetic protein-2 in cleft lip and palate patients. Tissue Eng Part C Methods. 2010 Oct;16(5):1183-9. DOI:
10.1089/ten.TEC.2009.0824
48. El Backly RM, Zaky SH, Canciani B, Saad MM, Eweida AM, Brun F, Tromba G, Komlev VS, Mastrogiacomo M, Marei MK, Cancedda R. Platelet rich plasma enhances osteoconductive properties of a hydroxyapatite-β-tricalcium phosphate scaffold (Skelite) for late healing of critical size rabbit calvarial defects. J Craniomaxillofac Surg. 2014 Jul;42(5):e70-9. DOI:
10.1016/j.jcms.2013.06.012
49. Dutra CE, Pereira MM, Serakides R, Rezende CM. In vivo evaluation of bioactive glass foams associated with platelet-rich plasma in bone defects. J Tissue Eng Regen Med. 2008 Jun;2(4):221-7. DOI: 10.1002/term.86
50. Luaces-Rey R, Arenaz-Búa J, Lopez-Cedrún-Cembranos JL, Herrero-Patiño S, Sironvalle-Soliva S, Iglesias-Candal E, Pombo- Castro M. Is PRP useful in alveolar cleft reconstruction? Platelet- rich plasma in secondary alveoloplasty. Med Oral Patol Oral Cir Bucal. 2010 Jul;15(4):e619-23.
51. Canan LW Jr, da Silva Freitas R, Alonso N, Tanikawa DY, Rocha DL, Coelho JC. Human bone morphogenetic protein-2 use for maxillary reconstruction in cleft lip and palate patients. J Craniofac Surg. 2012 Nov;23(6):1627-33. DOI:
10.1097/SCS.0b013e31825c75ba
52. Fallucco MA, Carstens MH. Primary reconstruction of alveolar clefts using recombinant human bone morphogenic protein-2:
clinical and radiographic outcomes. J Craniofac Surg. 2009 Sep;20 Suppl 2:1759-64. DOI:
10.1097/SCS.0b013e3181b5d08e
53. Neovius E, Lemberger M, Docherty Skogh AC, Hilborn J, Engstrand T. Alveolar bone healing accompanied by severe swelling in cleft children treated with bone morphogenetic protein-2 delivered by hydrogel. J Plast Reconstr Aesthet Surg. 2013 Jan;66(1):37- 42. DOI: 10.1016/j.bjps.2012.08.015
Corresponding author:
Dr. Helmy Soliman
Plastic Surgery Department, Mataria Teaching Hospital, GOTHI, Cairo, Egypt
helmysoliman@yahoo.com
Please cite as
Soliman H, Al-Kandari Q, Ismail H, Shouman O, Bahaa El-Din A, El Hadidy M. Adipose-derived stem cells in alveolar cleft management:
looking for a better scaffold. GMS Ger Plast Reconstr Aesthet Surg.
2018;8:Doc01.
DOI: 10.3205/gpras000049, URN: urn:nbn:de:0183-gpras0000491
This article is freely available from
http://www.egms.de/en/journals/gpras/2018-8/gpras000049.shtml Published:2018-07-02
Copyright
©2018 Soliman et al. This is an Open Access article distributed under the terms of the Creative Commons Attribution 4.0 License. See license information at http://creativecommons.org/licenses/by/4.0/.