Review article
e-Neuroforum 2016 · 7:71–81 DOI 10.1007/s13295-016-0032-4 Published online: 29 November 2016
© Springer-Verlag Berlin Heidelberg 2016
Carsten Duch · Stefanie Ryglewski
Institute of Zoology, Neurobiology, Johannes Gutenberg-University Mainz, Mainz, Germany
Structure and function of neuronal dendrites
Dendrites
The nerve cell counts as an elemen- tary unit of information processing in the brain, and neuronal networks evolve through synaptic connections be- tween nerve cells. Dendrites are highly branched structures of nerve cells that are specialized for receiving and process- ing synaptic input [1]. Hence, dendrites represent the structural substrate for synaptic inputs onto nerve cells and are the blueprint for synaptic connectivity in neuronal networks. As a matter of principle it is assumed that large network computing power is represented by large numbers of nerve cells and synapses.
Thus, in the human brain an estimated 100 bn nerve cells contact more than 150,000 kilometers of dendrites through more than 1015 synapses [2]. Further- more, dendritic structure defects provide anatomical correlates of neurological dis- eases such as for example Alzheimer’s, schizophrenia, Down syndrome, clinical anxiety as well as Rett syndrome and other autism spectrum disorders [3,4].
However, in most cases it remains un- clear in how far the anatomical dendritic structure defect is the cause or a con- sequence of the cognitive disorder as neuronal dendrites are multifunctional and are still insufficiently understood.
In the face of the vast variety of dendritic architectures in different types of neurons, the multi-functionality of dendrites of functionally different neu- rons seems obvious. In fact, neurons are classified based on their dendritic morphology (.Fig.1). Although mor- phological variability between neurons of the same type definitely exists, the molecular identity of a neuron defines its dendritic morphology to a certain
extent. Hence, medium spiny neurons of the basal ganglia (.Fig.1a) and cerebel- lar Purkinje neurons of mouse and rat (.Fig.1b) as well as cortical pyramidal neurons in mouse, rhesus monkey, and human (.Fig.1c) each look more alike than all three different neuron types in mouse (.Fig.1). This neuron-type- specific dendritic architecture even re- mains unchanged to a certain degree after new outgrowth in primary cell culture following dissociation, implying that this feature is, at least in part, ge- netically determined. Since the neurons shown in .Fig.1 have entirely differ- ent functions in the basal ganglia, the cerebellum, and cortex and since these functions correlate with their respec- tive characteristic morphologies, it is obvious to assume that dendritic struc- ture contributes significantly to neuron- type specific function. But what are the different roles of different dendritic morphologies?
Morphological and functional diversity of dendrites
First, dendrites expand the receptive sur- face of a neuron by 10- to 20-fold [5]. One obvious function of highly branched den- dritic structure is also an increase of the number of possible input synapses and, hence, likely computing power. It was shown that in sympathetic ganglion cells dendritic length correlates linearly with the number of input synapses [6], but be- cause of space and cost issues total den- dritic length and synapse number can- not be increased arbitrarily. Inspection of cortex suggests a construction prin- ciple in which the total dendritic length is minimized on the basis of the given number of synapses [7].
Second, the density of coverage of in- put space of a neuron varies depending on its dendritic structure. Although on average the dendrites of medium spiny neurons in mouse (.Fig.1a) reach at least as far as the ones of mouse Purkinje neurons (.Fig.1b) and therefore cover a similar neuropil volume, the latter ex- hibit 10 to 30 times more branches and have a substantially larger total length.
Accordingly, a Purkinje cell in cerebellum receives approximately 100,000 synaptic inputs from cerebellar climbing fibers, whereas the density of input synapses onto medium spiny neurons is estimated to be only about 15 synapses per 10 μm [8] which corresponds to a total of about 2000–6000 synaptic inputs. Hence, the much more dense branching pattern of Purkinje cells in comparison to medium spiny neurons can likely be explained by the number of input synapses.
Third, naturally morphology does not only determine how many but also which neurons contact a specific dendrite and therefore the logic of connections in neuronal networks. Considering for ex- ample the anatomically separated apical and basal dendritic subtrees of pyramidal neurons (.Fig.1c, green and pink), one could conclude that this may serve the segregation of various synaptic signaling pathways into different synaptic input domains of the same cell. This could sim- ply be a consequence of the distribution of diverse synaptic partners at different locations, like for example in various cor- tical layers that are covered by Pyramidal neuron dendrites. On the other hand, differential modulation of disparate in- puts may allow the separate influence of varying types of inhibitory interneurons or different synaptic learning events at various locations throughout a neuron
Fig. 18Dendritic morphology classifies neuron types. Examples of typical dendritic architectures of three different types of nerve cells from different mammals. All neuron reconstructions are from the Neuromorph database (NeuroMorpho.org).
The respective Neuromorph identification numbers and original references are provided underneath the respective neurons.
aMedium spiny neuron from the basal ganglia of mouseand rat.bCerebellar Purkinjeneurons from mouse and rat. All neurons inaandbare on the same scale.cCortical pyramidal neurons from mouse, rhesus monkey, and human.Scale barincis 7-fold smaller than inaandb
[5]. In Pyramidal neurons domain- specific synaptic input, modulation, and excitability appear functionally critical for coincidence detection, synaptic inte- gration, and plasticity [9]. In short, the functioning of dendrites reaches from the appropriation of sufficient surface for synaptic inputs to highly compartmen- talized units for molecular signaling and electrical computation. The situation becomes even more complicated due to dendrites not only functioning as passive recipients of synaptic information, but they may also possess output synapses and non-linear voltage gated ion channels which enormously increases the theoret- ically possible computational power of a single neuron [10–12]. Therefore, ulti- mately the specific function of dendritic architecture needs to be investigated for every neuron-type. However, basic rules of dendritic integration that in principle apply to all neuron types definitely exist, but depending on a neuron’s morphology and ion channel repertoire these rules may impart different functions.
Filtering of synaptic input in dendrites
The backbone of synaptic integration in all neurons is the passive dendritic archi- tecture, even if the computational power
of dendrites may be augmented and mod- ified significantly by the expression of voltage gated ion channels. Most neu- rons encode information by the rate and timing ofaction potentials. Usuallysingle excitatory synaptic inputs do not suffice to bridge the difference between resting membrane potential and firing thresh- old. Hence, in dendrites mostly many synaptic inputs need to be summed up to allow generation of an action potential.
The temporal and spatial summation of synaptic inputs depends decisively on the passive architecture of the dendritic ar- bor. Forty years ago Wilfrid Rall started to formally describe the passive features of dendrites in a series of modeling stud- ies using three cable properties: the axial resistance (Ra), the membrane capaci- tance (Cm), and the membrane conduc- tance (Gm) or membrane resistance (Rm= 1/Gm), respectively. The flux of a synap- tic current directly at the position of an input synapse in a dendrite causes a lo- cal change of the membrane potential that depends upon the geometry and the three abovementioned cable properties [13–15]. The key point is, however, what impact this local postsynaptic potential (PSP) has on the membrane potential at the action potential initiating zone. For demonstration purposes we assume that the action potential is generated in or
close by the soma, as is the case in many vertebrate neurons, and that two differ- ent local synaptic potentials with varying distance from the soma cause identical local dendritic EPSPs (.Fig.2a, synapse 1 blue, synapse 2 green). In a passive den- dritic cable the axial resistance Raand the membrane conductance Gmcause a con- tinual decrease of the PSP amplitude on its way to the soma. Since in the example shown in.Fig.2athe distal synapse 1 is further away than the proximal synapse 2, a single activation of the distal synapse 1 results in a smaller depolarization at the soma (.Fig.2a). Thus, in passive den- drites the effective amplitudes of PSPs de- crease with the distance to the soma. Fur- thermore, the membrane capacitance Cm
lengthens the time course of the synaptic potential. Since Cmincreases with an in- creasing membrane surface, distal synap- tic inputs are temporally stretched more strongly than proximal ones (.Fig.2a).
Consequently, filtering in passive den- drites transforms a short and sharp PSP intoa much smallerand broaderelectrical signal on its way to the soma (.Fig.2a).
Therefore, single synaptic inputs in purely passive dendrites should be less effective.
On the other hand, dendritic filtering may lengthen the time window in which the activation of distal synapses overlaps with other inputs. This may increase the
period in which effective temporal sum- mation may occur (.Fig.2a). We reca- pitulate that dendritic filtering may lead to smaller somatic PSPs produced by dis- tant synapses which may in turn be sub- ject to temporal summation over longer time periods. Already Rall’s theoretical contemplations [13–15] predict that the various functions of input synapses in passive dendrites can be distinguished by their location.
Proximal inputs lead to temporally narrow responses with large amplitudes and may therefore act as coincidence de- tectors, whereas distal inputs may serve temporal integration [16].
Democratization of effective amplitudes of synaptic inputs
Filtering properties of dendrites may as well be subject to alteration or be disinte- grated altogether (.Fig.2b, c). In many dendrites the local PSPs at different lo- cations are not identical as suggested in
.Fig.2a, but the amplitudes of postsy- naptic potentials upon identical synaptic currents vary depending on where the synapse is (.Fig.2b). Formally, the am- plitude of a local postsynaptic potential at the location of a postsynaptic current is a function of the input resistance at this very location. The input resistance, in turn, is a function of the membrane resistance, the axial resistance, and the geometry of the dendrite. The evoked difference of the local potential is larger the less current is shunted through neigh- boring dendrites, meaning the higher the specific axial (Ra) as well as the specific membrane resistances (Rm) are at this location. Rmand Raincrease along with a decrease of the diameter of the cable, but they are also affected by the branch- ing structure. Since in many neurons the diameter of dendritic branches decreases more distally, the local input resistance increases with the distance from the soma or the action potential initiating zone (.Fig.2b). This means that distal synap- tic currents (.Fig.2b, blue synapse) re- sult in larger local membrane potential changes than proximal ones (.Fig.2b, green synapse). As distal PSPs attenu- ate more strongly on their way to the soma these two effects act complemen-
Abstract · Zusammenfassung
e-Neuroforum 2016 · 7:71–81 DOI 10.1007/s13295-016-0032-4
© Springer-Verlag Berlin Heidelberg 2016
C. Duch · S. Ryglewski
Structure and function of neuronal dendrites
Abstract
Neurons represent the cellular substrate for information processing in the nervous system. Already around 1900 the Spanish neuroanatomist Ramón y Cajal proposed that neurons possess two discrete functional domains, the axonal and the somatodendritic compartment. Cajal established the founda- tion for the neuron doctrine by suggesting dendrites to be the synaptic input regions of neurons, and that information processing travels from dendritic regions towards axon terminals and output synapses (“the theory of dynamic polarization”, Shepherd, 1991).
Despite a number of exceptions, for most neurons this rule prevails to the present.
Therefore, dendritic architecture has two fundamental functions in the nervous system.
First dendrites expand the receptive surface of neurons, and their shape dictates how many and which presynaptic neurons can contact a postsynaptic dendritic arbor.
Thus, dendritic structure influences the number of synapses as well as the wiring logic within neuronal networks. Second dendritic structure impacts the temporal and spatial integration of postsynaptic potentials.
Accordingly, in different types of neurons with different functions dendritic gestalt differs significantly, and dendritic architecture often serves to classify neuron types. In most cases, however, the specific function of dendritic architecture remains largely elusive. Dendritic structure analysis is further bedeviled by dendrites exhibiting voltage-gated ion channels which themselves vastly modify function and computing power. Although a multitude of neurodevelopmental and
neurodegenerative disorders coincides with dendritic defects, it often remains unclear whether these structural defects are the cause or a consequence of the dysfunction.
Therefore, on the one hand it is important to determine the contribution of dendritic structure to the function of different types of healthy neurons. On the other hand the question arises whether dendritic defects impact neuronal function qualitatively and to what degree of dendritic defect neuronal function can be maintained. This article will first summarize basic functions of passive dendritic architecture which applies for most neurons but confers variable characteristics to different types of neurons. It will be discussed how the location of input synapses in a passive electrical structure affects the integration of postsynaptic potentials.
Then principles will be introduced how this localization-dependence of synaptic inputs into dendrites can be compensated for. And finally, an identified Drosophila motoneuron will serve as an example that at least in specific types of neurons basic function can be maintained with a minimum number of dendrites and input synapses. By contrast, in this example dendritic structure is imperative for fine tuning of adaptive behavioral functions which are essential for survival and reproduction. These findings will then be discussed in the context of other neuronal functions.
Keywords
Dendrite · Motorneuron · Synaptic integration · Structure-function relation
tary. Larger local PSPs in distal dendrites and stronger attenuation of a signal on its way to the soma may be balanced in a way that the effective PSP amplitudes at the soma are identical for distal and prox- imal synaptic inputs (.Fig.2b). This can even go thus far that synaptic inputs from all dendritic input sites elicit the same effective PSP amplitude at the action po- tential initiating zone, namely if for all synaptic sites in dendrites the decrease of the PSP amplitude over the distance is inversely proportional to the input re- sistance. This construction principle has been described in depth by Cuntz et al.
[17] in detailed multicompartment mod-
els using realistic geometries as well as passive biophysical properties of tangen- tial cells of the fly visual system. Our own data using passive models of flight motoneurons ofDrosophila melanogaster support this principle of synaptic democ- racy (see.Fig.4).
Other mechanisms exist to amplify the effective PSP amplitudes of distal synapses. First, distal input may be boosted by voltage dependent calcium and sodium channels. However, ampli- fication through voltage gated channels does not appear to play a role until many synapses are activated because these channels only open at strongly
Fig. 28Dendritic filtering. Schematic drawing of some properties of dendritic integration of synap- tic inputs.aAmplitudes and time courses of postsynaptic potentials (PSPs) at the soma (or the action potential initiating zone) differ due to the passive filtering properties of dendrites depending on the distance of the input synapse (left: sketch drawing of distal input synapse1,blue, and proximal input synapse,green, relative to the soma). Thetop two tracesof the second column depict the respective PSPs at the synapse at equivalent synaptic current amplitudes and equivalent local input resistances.
Thebottom traceshows the resulting PSPs at the soma following activation of synapse 1 or 2. The third column represents PSPs at repetitive stimulation at the proximal synapse 2 (green) and the following temporal summation at the soma (bottom trace). Column four exhibits PSPs at repetitive stimulation at the distal synapse 1 (blue) and the resulting temporal summation at the soma (bottom trace). Tem- poral summation as observed at the soma is larger for the distal synapse.bThe varying somatic PSP amplitudes may be compensated for either by gradually increasing input resistances from proximal to distal, or by gradually enhanced synaptic currents. However, this does not apply for the varying tem- poral summation of distal and proximal inputs.cDiffering temporal summation as caused by distal or proximal synaptic inputs can be compensated for by gradually increased dendritic ion channels from proximal to distal (e. g. HCN channels)
depolarized membrane potentials, and their activation is counteracted by out- wardly directed potassium currents [18].
Second, and already of great importance with the activation of single synapses, evidence from pyramidal cells exist that synaptic strength is scaled with the dis- tance from the soma so that dendritic filtering of PSP amplitudes on their
way to the soma is counterbalanced [16]. In principle, synaptic transmis- sion strength with cumulative distance from the soma may be caused either by elevated transmitter release at distal presynapses or by increased postsynaptic conductance (.Fig.2b, Gsyn). Studies using CA1 pyramidal neurons suggest that the postsynaptic receptor density
scales with the distance from the soma so that distal inputs cause larger local PSPs [16, 19]. However, another unsolved problem is how the distance of a synapse to the soma is encoded so that it can be scaled accordingly. The construction principle of increasing input resistance in distal dendrites (inversely proportional to the decrease of PSP amplitude on its way to the soma) as well as the scaling of synaptic strength (elevated in distal dendrites) lead to democracy of the effective PSP amplitudes of all synaptic input sites but they do not affect the time course of the PSP (.Fig.2a, b). The time course is slackened the more membrane is between the synaptic input site and the soma. Thus, the independence of the location of the PSP amplitudes does not necessarily affect the time window in which distal inputs are summed up (.Fig.2a, b).
Democratization of the temporal integration of synaptic inputs
Certainly, in many neuron types, includ- ing pyramidal neurons, the width of so- matically recorded PSPs does not de- pend upon the location of the input site.
This means the temporal summation is vastly the same for all excitatory input synapses [16]. In purely passive den- drites the time course of postsynaptic potentials depends upon the time con- stant (τ) of the membrane which in turn is the product of membrane resistance (Rm) and membrane capacitance (Cm).
An increased time constant as a result of an increased membrane capacitance for distal synapses may, in principle, al- ready be counterbalanced by an increas- ing density of open ion channels and, thus, a decreased membrane resistance (.Fig.2c). In pyramidal neurons this is realized by a gradient of HCN channels, with an increasing channel density from proximal to distal (.Fig.2c). HCN chan- nels conduct sodium and potassium ions, open upon hyperpolarization, and a sig- nificant number of HCN channels are open at the resting membrane potential [20]. This leads to a decrease of the mem- brane resistance in dendrites from prox- imal to distal because of the HCN chan-
nel gradient. In addition, HCN channels inactivate following local membrane de- polarization. The resulting reduction of sodium influx causes a hyperpolariza- tion that shortens the synaptic depolar- ization and, therefore, the duration of the EPSP. Thereby, the HCN channel gradi- ent mediates a location-independence of the temporal summation of excitatory synaptic inputs. Although further active mechanisms exist, at least in pyramidal neurons HCN channels pose the most important mechanism to adjust tempo- ral summation for all synaptic input sites because pharmacological block of HCN channels cause a significant broadening of PSPs from distal synapses [16]. The strategic placement of voltage dependent ion channels in dendrites may therefore compensate for temporal filtering prop- erties of passive dendrites and transform distal inputs conveying sustained tempo- ral summation into shorter inputs used for coincidence detection. The combina- tion of active conductances with different dendritic morphologies and the strategic localization of excitatory and inhibitory synapses permits a vast multiplicity of computational actions in various types of neurons [18,21] that will not be dis- cussed any further here. In this article we will mainly focus on the dendrites of motoneurons innervating skeletal mus- cles.
Dendrites of motoneurons
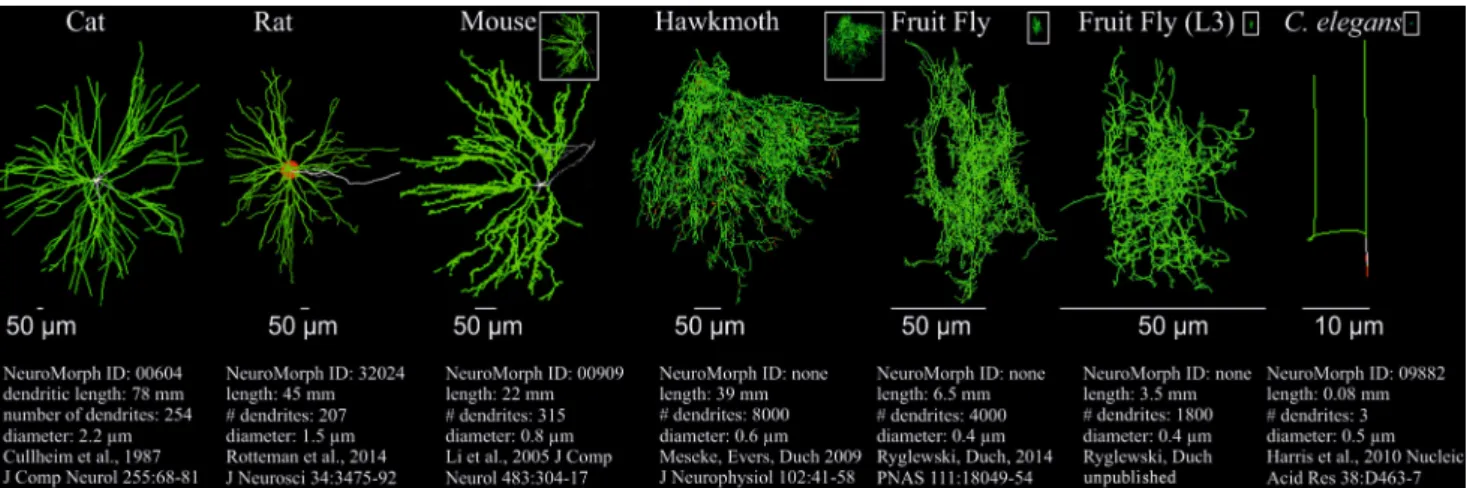
In vertebrates the so-called lower mo- toneurons (LMNs) are located in the ven- tral horn of the spinal cord innervating skeletal muscles via the spinal nerves. In insects most motoneurons innervating skeletal muscles are in the ventral nerve cord (VNC) or ventral nervous system (VNS)..Fig.3depicts motoneurons of three different vertebrate species, of three insects, and one pharyngeal motoneuron of the nematodeC. elegans. An exten- sive dendritic tree is a commonality in all skeletal motoneurons, whether in ver- tebrates or insects, without any obvious subdivision into different domains. Mo- toneuron dendrites are distributed more or less evenly across the motor neuropils.
However, it is known that inhibitory input synapses in vertebrate spinal motoneu-
rons as well as in flight motoneurons of the fruit fly,Drosophila melanogaster, mainly target dendrites that are proximal to the action potential initiating zone (cat, [22]; Drosophila, [23]). Consequently, temporal summation (.Fig.2) is shorter for inhibitory inputs as compared to exci- tatory inputs, meaning inhibition is usu- ally sharper in motoneurons than exci- tation. In contrast, excitatory inputs are localized as far out as the most distal den- drites. The spatial distribution of den- drites is enormous. It reaches well to the neuropil borders. In the example of cat in.Fig.3, spinal motoneuron dendrites reach 11 cm from the soma to the neu- ropil borders. Because of the dendritic fil- tering properties, in entirely passive den- drites distal excitatory inputs would only elicit very small effective amplitudes in the soma, and thus, not contribute sig- nificantly to action potential initiation.
However, in vertebrate motoneurons the amplitudes of excitatory input are en- hanced by dendritic voltage gated cur- rents. The activation of persistent in- ward currents, mostly by L-type calcium channels and sodium channels, results in amplification of excitatory postsynaptic potentials. Depending on the excitation status of the animal, this amplification of excitatory PSPs is modulated by descend- ing aminergic neurons in brain stem [24].
In addition, it becomes clear that the total dendritic length of vertebrate motoneu- rons scales with the size of the animal and, hence, the diameter of the spinal mo- tor neuropils (.Fig.3; dendritic length spinal motoneuron of cat: 78 mm; rat:
45 mm; mouse: 22 mm). Correspond- ingly, also the average dendritic diame- ters scale (.Fig.3) which likely further counterbalances the attenuation of dis- tal PSPs over large distances. Since the ventral nervous system of insects is usu- ally smaller as compared to the spinal cord of vertebrates, the spatial dimen- sions of insect motoneurons are signifi- cantly less widespread. In.Fig.3scales are matched for better visualization. The white boxes depict the spatial dimen- sions of the respective motoneurons but are scaled to the cat motoneuron. Al- though the spatial dimensions of den- drites in small insects are vastly smaller, the total dendritic length does, by no
means, scale as compared to vertebrates.
For example, the dimensions of the flight motoneuron of the fruit fly,Drosophila melanogaster (.Fig.3, example 5), are five times smaller as compared to mouse (.Fig.3, example 3), but its total den- dritic length is larger by a factor of three (6.5 mm vs. 2.2 mm). The reason is that insect motoneurons often exhibit vastly more dendritic arborizations. The large spinal motoneuron of cat has a total den- dritic length of 78 mm but possesses only 254 single dendritic branches (.Fig.3, example 1). By contrast, the flight mo- toneuron of the hawkmoth, Manduca sexta(.Fig.3, example 4) has a total den- dritic length of 39 mm but holds a total of 8000 single dendritic branches. So, usu- ally insect motoneurons are way more branched than vertebrate motoneurons, and therefore, cover the neuropil signif- icantly more densely (.Fig.3). Addi- tionally, in relation to vertebrates they show an increased total dendritic length relative to their marginal spatial extent.
Accordingly, despite their small dimen- sions insect motoneurons face the same dendritic filtering problems like spinal motoneurons. The solution to this prob- lem appears to be similar in insects and vertebrates. Also in fruit fly motoneurons we find dendritically localized calcium channels [25, 26] that boost excitatory postsynaptic potentials and are subject to modulation by aminergic inputs (Ry- glewski et al., unpublished).
In insects, motoneuron dendrites scale with the size of the animal and, thus, the dimensions of the motor neu- ropils in the ventral nervous system, as in vertebrates. In the examples 4 and 5 in.Fig.3the total dendritic length of the wing depressor motoneuron, MN5, inManduca sexta (.Fig.3, example 4) and inDrosophila melanogaster(.Fig.3, example 5) is about twice the body size of the respective animal. The example mo- toneuron of the tinier Drosophila larva exhibits a lower total dendritic length.
Overall, as compared to vertebrates, insect motoneurons are more complex, possess significantly more arborizations, and have an enormously enhanced total dendritic length in relation to their body size. Although this assumption is spec- ulation, a possible reason may be that
Fig. 38Dendritic structure of motoneurons of vertebrates, insects, andC. elegans.Comparison of the dendritic structures of cat, rat, and mouse spinal motoneurons, insect (Manduca sextaadult;Drosophila melanogasteradult and larva) skeletal muscle motoneurons, and aC. eleganspharyngeal motoneuron (in this order, fromlefttoright). Due to the different scaling, thescale barsare adjusted accordingly. Thewhite boxesdepict the respective motoneurons at the same scale as the one of cat.
Total dendritic length, number of dendritic branches, and average dendrite diameters are provided underneath the respective reconstructions. The three vertebrate and theC. elegansmotoneuron reconstructions are from the Neuromorph database (NeuroMorph.org). The respective Neuromorph identification numbers are provided underneath the respective neurons
insect motoneurons must process rela- tively more synaptic information. First, graded muscle contraction in insects is often not realized by the activation of various motor units but by graded synaptic transmission onto the muscle.
Second, insect muscles are innervated only by a small number of motoneurons but their motor performance is immense.
As opposed to the complex motoneu- ron dendrites in vertebrates and insects, C. eleganspharyngeal motoneuron den- drites exhibit only a minimal structure (.Fig.3). It is worth mentioning, how- ever, thatC. eleganspossesses only 302 neurons and few muscles.
In order to probe the function of the highly branched dendrites of insect or vertebrate motoneurons directly, they need to be manipulatedin vivoto then test the consequences for the resulting neuronal activity patterns and motor be- havior. In the following this is introduced using theDrosophila melanogasterwing depressor motoneuron, MN5 (.Fig.3, example 5).
The identified flight
motoneuron, MN5: a case study
MN5 is one of five motoneurons (.Fig.3, examples 4 and 5, Manduca sexta and Drosophila melanogaster, respectively) that innervate the dorsolongitudinal
wing depressor muscle (DLM). In Drosophila melanogaster the DLM de- pressor muscle mediates wing down stroke during flight and courtship song, the latter being the love song with which the male courts the female fly. There- fore, the function of MN5 during motor behavior is well known. The DLM is an asynchronous flight muscle, mean- ing that MN5 fires one action potential only about every 10thto 20thwing beat to tune the calcium concentration in this otherwise stretch-activated asyn- chronous muscle [27]. This means that MN5 fires tonically at about 5–10 Hz at wing beat frequencies of approximately 200 Hz. The firing rates of all five DLM motoneurons are regulated conjointly, and alterations of these firing rates are directly and linearly related with mod- ifications of the wing beat frequency and amplitudes, thereby directly regu- lating power output during flight [27].
Thus, dipteran depressor motoneurons do not influence the temporal fine- adjustment of the wing beat but very much the contraction frequencies and amplitudes of the large flight muscles.
During courtship song, two alternating song motifs are distinguished: the pulse and the sine song. During sine song the wings are waved up and down at low amplitude and a frequency of about 160 Hz, whereas pulse song is character-
ized by single large amplitude wing beats and species specific interpulse interval (~34 ms in Drosophila melanogaster).
During sine song only a subset of the DLM motoneurons are activated at a low frequency, whereas during pulse song all DLM motoneurons fire simultaneously at a higher frequency [28]. The activa- tion patterns of MN5 during flight and during courtship song are therefore suf- ficiently well known. The firing patterns can be recordedin vivoduring behavior from the target muscle fiber using fine tungsten wires, and the genetic tools in Drosophila offer the possibility to selec- tively manipulate only the dendrites of the DLM motoneurons without affecting other properties or other neurons [29].
This provides the prerequisite to now test the function of dendritic architecture of identified insect motoneurons directly in the context of behavior.
Location-independence of synaptic input in a Drosophila DLM motoneuron
The dendritic structure of vertebrate and insect motoneurons does not reveal any obvious domains (.Fig.3). Nonetheless, it appears important to obtain an estimate whether excitatory synaptic inputs onto different parts of the dendrite are biased based on dendritic filtering, meaning that
Fig. 48Location-independence of PSP amplitudes in the passive geometry of an identified Drosophila motoneuron.aDis- tribution of the local input resistances in a passive multicompartment model based on realistic morphology and passive mem- brane properties as recordedin situfrom the Drosophila wing depressor motoneuron MN5. Input resistances between 80 and 1000 MΩand are color-coded. Input resistances increase from proximal to distal.bColor-code of the distribution of the ratio of PSP amplitudes at the action potential initiating zone (VAP,white arrow) relative to the local PSP amplitudes at the respective dendritic branches (Vdendrite). VAP/Vdendritedecreases from proximal to distal.cRelative PSP amplitudes at the action potential initiating zone are similar for all dendritic branches due to the inverse proportionality of the ratio of input resistance and PSP attenuation.dBecause of the passive structure of the dendrite the local input resistances are inversely proportional to the PSP attenuation (VAP/Vdendrite). Multicompartment models modified after Berger [30]
specific areas of the dendrite need to be manipulated to be able to unravel the function. Based on multicompartment models that rely on realistic dendritic ar- chitecture as well as passive biophysical properties of MN5 as recordedin situ, hint at location-independence, at least with regard to the filtering of PSP am- plitudes in dendrites (.Fig.4, modified after Berger [30]). Plotting the distribu- tion of input resistances over all dendritic branches as a color code (.Fig.4a), it be- comes obvious that the input resistance Rinputincreases in higher order distal den- drites and, thus, the local PSP amplitudes in these dendrites are larger following the same synaptic currents as compared to proximal dendrites (compare.Fig.2b).
Now, the decline of PSP amplitudes on their way to the action potential initiat- ing zone (.Fig.4b, VAP/Vdendrite) behaves exactly the opposite. In MN5 the action
potential is not initiated at the soma but at the primary neurite (.Fig.4, white arrow). In fact, for all dendritic loca- tions the decline of the PSP amplitude on its way to the action potential initi- ating zone is inversely proportional to the respective specific input resistances (.Fig.4d). Thereby, at least for a sin- gle current injection in every dendritic branch the effective PSP amplitudes are basically the same at the action poten- tial initiating zone (.Fig.4c). In addi- tion, with regard to temporal summa- tion of excitatory inputs several dendritic conductances mediate a certain location- independence (Ryglewski, unpublished).
Therefore, a selective manipulation of specific parts of the dendritic tree does not seem imperative for the analysis of the function of this motoneuron’s dendritic structure.
The function of the dendritic structure of an identified Drosophila motoneuron
Drosophila offers the opportunity to spatially restrict expression of RNAi transgenes to only few motoneurons using the binary GAL4/UAS expression system. With the use of specific GAL4 drivers only the DLM wing depressor motoneurons are addressed without affecting other neurons. However, to selectively test the function of dendritic architecture a manipulation is needed that only encumbers intrinsic dendrite growth but does not influence any other physiological properties of those neu- rons and that also does not indirectly affect other neurons. Targeted RNAi knock down of the cell surface molecule Dscam1 (Down syndrome cell adhesion molecule 1) yields a massive dendritic
phenotype in the DLM motoneurons [31]. For MN5 this means that the den- drites do not reach the neuropil borders anymore, and higher order branches are entirely missing. The total dendritic length is reduced by more than 90%, from 6 mm to below 600 μm (.Fig.5a, b). This manipulation does not affect the axonal innervation of the target DLM muscle fiber, and neuromuscular transmission remains unaltered [29]. Furthermore, the basically dendrite-less MN5 exhibits the same potassium, sodium, and cal- cium currents per membrane surface as their wildtype controls. And finally, even following a 90% reduction of dendrites, MN5 still receives excitatory cholin- ergic and inhibitory GABAergic input synapses [29]. The conclusion is that a targeted Dscam1 RNAi knock down only in the DLM motoneurons selectively reduces their dendritic length by more than 90% but leaves all other properties of these neurons intact. This opens up the possibility to use targeted Dscam1 RNAi knock down to now selectively test the function of MN5 dendrites in the context of behavior.
What can a motoneuron still do after losing more than 90% of its dendrites?
Strikingly, even with virtually dendrite- less wing depressor motoneurons fruit flies are still able to fly (.Fig.5c), with MN5 showing its typical tonical firing pattern during flight. This allows the conclusion that a sufficient number of presynaptic partner neurons still contacts MN5 in a correct manner even though MN5 dendrite coverage of the neuropil is minimal. In controls MN5 dendrites cover a volume of neuropil of approxi- mately 120 × 103μm3(.Fig.5a) but only about 12 × 103μm3, all close to the pri- mary neurite, following Dscam1 RNAi knock down (.Fig.5b). Nevertheless, during development a sufficient number of axon terminals target the remaining MN5 dendrites to successfully integrate it into the flight network. This suggests that during pathfinding and synaptoge- nesis the presynaptic partners grow to- wards the motoneuron dendrites and not the other way around. Following Dscam1 knock down, however, MN5 appears to be equipped with fewer excitatory input synapses because it fires with a signif-
icantly lower frequency which in turn reduces wing beat frequency and flight time (.Fig.5c) which does not seem to affect normal flight performance. With this the question arises why the flight mo- toneurons in wildtype flies exhibit more than 6 mm of dendrites. This becomes obvious if the flies are assigned challeng- ing motor tasks. Fruit flies modulate flight performance upon optomotor in- put. Presenting flies with a high contrast horizon in their visual field and mov- ing it up fools the fly into the illusion it would be losing altitude during flying (.Fig.5f). Vice versa, moving the hori- zon down mediates the illusion of climb flight. The animals compensate for such differences in altitude with changing the flight muscle power output [27]. During descent the motoneuron firing frequency is increased (.Fig.5d) so that flight mus- cle contraction frequency and amplitude are enhanced. Such optomotor adjust- ments of flight performance by modu- lating motoneuron firing frequency are impossible following the loss of dendrites (.Fig.5e). Quantification reveals that in MN5 with 90% fewer dendrites the firing rate can be adjusted less strongly by a fac- tor of three and significantly slower as compared to control (.Fig.5g, d, e). This implies that complex dendritic structure is not imperative for basic motoneuron functioning but very much so for adap- tive motor performance. It is obvious that there is a high selection pressure on exquisite maneuverability during flight in dipterans.
The analysis of the role of mo- toneuron dendrites during Drosophila melanogastercourtship song makes this even clearer. As described above, DLM motoneurons are not only used during flight but also for wing movement during courtship song (.Fig.5h). The male’s love song during the courtship ritual can be recorded with sensitive microphones, thus, illustrating that flies with den- drite-less wing depressor motoneurons sing just like wildtype flies do during courtship (.Fig.5i), and both song mo- tifs, sine and pulse song, are executed correctly (.Fig.5h). Amplitudes, fre- quency, and interpulse intervals are not affected by a loss of flight motoneuron dendrites [29]. Nevertheless, animals
with dendrite defects in DLM motoneu- rons exhibit a four times reduced mating success (.Fig.5i). Therefore, motoneu- ron dendrites seem to be essential for mating success, at least in this experi- ment. This observation may well explain the huge investment in more than 6 mm dendrites in wildtype motoneurons, be- cause obviously mating success also means high selection pressure on den- dritic structure. But what distinguishes the songs of animals with or without dendrites in the DLM motoneurons?
Analysis of song records displays that both song motifs in themselves are ex- ecuted correctly [29]. However, the ratio of sine to pulse song in animals with dendritic defects is significantly increased (.Fig.5i). The conclusion is that dendritic defects result in the inability of the fly to switch from sine to pulse song in a normal way. Already in 1977 Ewing has shown that during sine song only few DLM motoneurons are active at a low firing frequency, and during pulse song all DLM motoneurons are active at a higher frequency [28].
This switch in the activation of the DLM motoneurons and, therefore, the quick change of song motifs seems impossible with an impaired dendritic structure.
Interestingly, the degree of dendritic defect scales with the magnitude of the functional impairment both with regard to the modulation of motoneuron fir- ing rates through optomotor input as well as the change between song motifs [29]. This demonstrates that complex dendritic structure does not determine the basic motor function or how an insect motoneuron is incorporated into a motor network. However, dendritic structure is essential for adaptive mo- tor performance that is imperative for mating success and survival.
Concluding remarks
Structure and function of neuronal den- drites are directly linked. Dendritic structure characterizes different neu- ron types, provides the blueprint for synaptic wiring in the nervous system, and filters synaptic inputs. Nonethe- less, it remains impossible to directly infer the function of dendrites from
Fig. 58The function of dendritic structure of an identified Drosophila wing depressor motoneuron.aThe identified wing de- pressor motoneuron MN5 is a unipolar neuron comprising more than 6mm of total dendritic length. The MN5 dendrites cover approximately 120 × 103μm3of the neuropil.bTargeted Dscam1 RNAi knock down only in the wing depressor motoneurons reduces the total dendritic length by more than 90% below 0.6mm and the neuropil coverage below 12 × 103μm3.cFlies with basically dendrite-less wing depressor motoneurons are able to fly but the motoneuron firing frequency and the wing beat frequency are reduced. However, a loss of dendrites does not fundamentally change motoneuron function or its integra- tion into the flight network.dMN5 firing frequency is subject to modulation by optomotor input.eOptomotor modulation of motoneuron activity is basically lost without dendrites.fBehavioral assay for optomotor stimulation.gQuantification of op- tomotor responses in dendrite-less vs. control motoneurons.hMale courtship song shows both song motifs, pulse and sine song, in animals with wing depressor motoneurons with and largely without dendrites. Sine and pulse songs are identical in the respective animals as is the relative song duration during courtship (i) but mating success is strongly decreased with- out dendrites (i). Following a loss of dendrites the courtship song is less successful because switching between song motifs is significantly more difficult (i). Figure is modified from Ryglewski et al. [29]
their architecture. Although the passive filtering properties of dendrites in all neurons follow the same rules, these filtering properties can be adjusted fun- damentally depending on ion channel equipment and varying synapse reper- toire. Consequently, for each neuron type function and computing power of dendritic structure must be determined individually. Also for the same types of neurons in different species, specific differences and commonalities exist. The present article aimed to show for one identified Drosophila motoneuron that a loss of structure does not come with a change in function but that it coincides with reduced computing power during adaptive behavior. This is not true for all neurons. For our example motoneuron it seems irrelevant which dendrites are affected since synaptic inputs largely display location-independence. In other cases, however, clustering of excitatory and inhibitory inputs to different den- dritic domains, for example, underlies specific computing power. A prominent example is an identified visual interneu- ron in the locust that encodes the time to collision with an object during flight that is progressively getting larger in the visual field. For this, differential excita- tory and inhibitory inputs into different dendritic domains are needed [32]. In this example a structural defect of such a dendritic domain would cause a false time-code. Hence, the function of this neuron would be altered fundamentally.
By contrast, structural dendritic defects in the abovementioned Drosophila wing depressor motoneuron do not impair its function, but the extent of this structural defect scales with decreased comput- ing power. This result is in accord with gradually diminished brain power with advancing dendritic defects during progressive neurological diseases. How- ever, generalization is delicate due to the diversity of dendritic structure, the compartmentalization into various func- tional domains, and the equipment with different combinations of ion channels.
Corresponding address
C. Duch
Institute of Zoology, Neurobiology, Johannes Gutenberg-University Mainz Col. Kleinmann Weg 2, 55099 Mainz, Germany cduch@uni-mainz.de
S. Ryglewski Institute of Zoology, Neurobiology, Johannes Gutenberg-University Mainz Col. Kleinmann Weg 2, 55099 Mainz, Germany ryglewsk@uni-mainz.de
Compliance with ethical guidelines
Conflict of interest.C. Duch and S. Ryglewski declare that they have no competing interests.
This article does not contain any studies with human participants performed by any of the authors. All ap- plicable international, national, and/or institutional guidelines for the care and use of animals were fol- lowed.
References
1. Fiala JC, Spacek J, Harris KM (2008) Dendritic structure. In: Stuart G, Sprouston N, Häusser M (eds) Dendrites. Oxford University Press, Oxford, pp 1–34
2. Azevedo FA, Carvalho LR, Grinberg LT, Farfel JM, Ferretti RE, Leite RE, Filho JW, Lent R, Herculano- Houzel S (2009) Equal numbers of neuronal and non-neuronal cells make the human brain an isometrically scaled-up primate brain. J Comp Neurol 513(5):532–541
3. Kaufmann WE, Moser HW (2000) Dendritic anomalies in disorders associated with mental retardation. Cereb Cortext 10(10):981–991 4. Kulkarni VA, Firestein BL (2012) The dendritic tree
and brain disorders. Mol Cell Neurosci 50:10–20 5. Mel BW (2008) Why have dendrites? A compu-
tational perspective. In: Stuart G, Sprouston N, Häusser M (eds) Dendrites. Oxford University Press, New York, pp 421–437
6. Purves D, Hume RI (1981) The relation of postsy- naptic geometry to the number of presynaptic axons that innervate autonomic ganglion cells.
J Neurosci 1(5):441–452
7. Wen Q, Stepanyants A, Elston GN, Grosberg AY, Chklovskii DB (2009) Maximization of the connectivity repertoire as a statistical principle governing the shapes of dendritic arbors. Proc Natl Acad Sci U S A 106(30):12536–12541
8. Huerta-OcampoI,Mena-SegoviaJ,BolamJP(2014) Convergence of cortical and thalamic input to directandindirectpathwaymediumspinyneurons in the striatum. Brain Strut Funct 219:1787–1800
9. Sprouston N (2008) Pyramidal neurons: dendritic structure and synaptic integration. Nature Rev Neurosci 9:206–221
10. Branco T, Häusser M (2010) The single dendritic branch as a fundamental functional unit in the nervous system. Curr Opin Neurobiol 20(4):494–502
11. Häusser M, Sprouston N, Stuart G (2000) Diversity and dynamics of dendritic signaling. Science 290:739–744
12. Koch C, Segev I (2000) The role of single neurons in information processing. Nat Neurosci 3:1171–1177
13. Rall W (1961) Distinguishing theoretical synaptic potentials computed for different soma-dendritic distributions of synaptic input. J Neurophysiol 30:1138–1168
14. Rall W (1967) Distinguishing theoretical synaptic potentials computed for different soma-dendritic distributions of synaptic input. J Neurophysiol 30:1138–1168
15. Rall W (1964) In: Reiss RF (ed) Neural Theory and Modeling. Standfort University Press, Palo Alto, pp 5–35
16. Magee JC(2000) Dendritic integration of excitatory synaptic input. Nat Rev Neurosci 190:181–190 17. Cuntz H, Borst A, Segev I (2007) Optimization
principles of dendritic structure. Theor Biol Med Model 8:4–21
18. Häusser M, Spruston N, Stuart GJ (2000) Diversity and dynamics of dendritic signaling. Science 290(5492):739–744
19. Magee JC, Cook EP (2000) Somatic EPSP amplitude is independent of synapse location in hipoocampal pyramidal neuron. Nat Neurosci 8:895–903 20. Biel M, Wahl-Schott C, Michalakis S, Zong X (2009)
Hyperpolarization-activatedcationchannels: from genes to function. Physiol Rev 89(3):847–885 21. London M, Häusser M (2005) Dendritic computa-
tion. Annu Rev Neurosci 28:503–532
22. Fyffe RE (1991) Spatial distribution of recurrent inhibitory synapses on spinal motoneurons in the cat. J Neurophysiol 65(5):1134–1149
23. Kühn C, Duch C (2013) Putative excitatory and putative inhibitory inputs are localised in different dendritic domains in a Drosophila flight motoneuron. Eur J Neurosci 37(6):860–875 24. Heckman CJ, Lee RH, Brownstone RM (2003)
Hyperexcitable dendrites in motoneurons and their neuromodulatory control during motor behavior. Trends Neurosci 26(12):688–695 25. Worrell JW, Levine RB (2008) Characterization of
voltage-dependent Ca2+ currents in identified Drosophila motoneurons in situ. J Neurophysiol 100(2):868–878
26. Ryglewski S, Lance K, Levine RB, Duch C (2012) Ca(v)2 channels mediate low and high volt- age-activated calcium currents in Drosophila motoneurons. J Physiol 590(4):809–825 27. Gordon S, Dickinson MH (2006) Role of calcium in
the regulation of mechanical power in insect flight.
Proc Natl Acad Sci 103(11):4311–4315 28. Ewing AW (1977) The neuromuscular basis of
courtship song in Drosophila: the role of the direct and axillary wing muscles. J Comp Physiol 130:87–93
29. Ryglewski S, Kadas D, Hutchinson K, Schuetzler N, Vonhoff F, Duch C (2014) Dendrites are dispensable for basic motoneuron function but essential for fine tuning of behavior. Proc Natl Acad Sci 111(50):18049–18054
30. Berger S (2014) Analysis of Signal Propagation and Excitability in Computational Models of an
Identified Drosophila Motoneuron. Dissertation, Arizona State University, 2014
31. Hutchinson KM, Vonhoff F, Duch C(2014) Dscam1 is required for normal dendrite growth and branch- ing but not for dendritic spacing in Drosophila motoneurons. J Neurosci 34(5):1924–1931 32. Gabbiani F, Krapp HG, Koch C, Laurent G (2002)
Multiplicative computation in a visual neuron sensitive to looming. Nature 420(6913):320–324