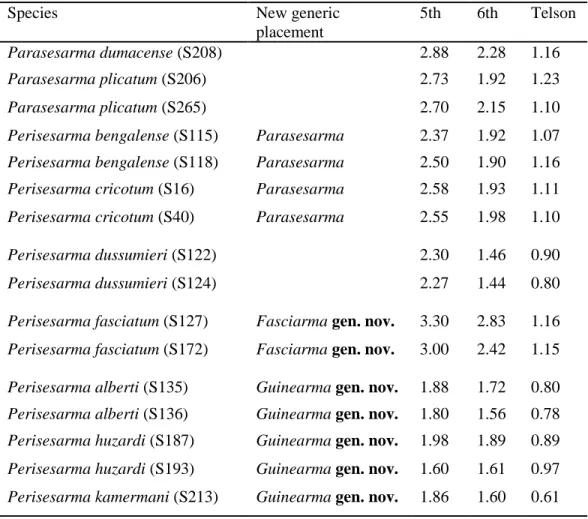
Molecular phylogeny, phylogeography and taxonomic revision of species of the genus Perisesarma De Man, 1895 (Crustacea: Decapoda: Brachyura: Sesarmidae)
Volltext
Abbildung



ÄHNLICHE DOKUMENTE
Sticherus pteridellus is characterized by having the 1 st axes as long as to slightly longer than the 2 nd , thin rhizomes with scattered, ovate, stoutly hyaline scales,
Legs mostly dark yellow ex- cept tarsus blackish brown; anterior face of femora and tibiae covered in black scales, posterior face of femora and tibiae covered in white
[r]
Redescription. Male: Body length 3 mm. Head: Eye bare. Frontal vitta brownish, reddish on anterior margin. Interocular space 0.4 mm. Two pairs of fr, with two in- tercalated
Thorax: Pronotum side fully punctate; anterior angles slightly acute; marginal groove on anterior margin occu- pying 1/3 of pronotum anterior border; median groove
Eyes dorsolaterally situated on head; eye diameter usually be- tween one-fourth and one-third the length of lateral ce- phalic margin in full-face view, rarely more; scape sur-
as long as wide; antennomere 9 moderately projecting apically, 1.3 × as long as wide; all elytral series weakly but sharply impressed; trochanters with- out tufts of yellowish
The male imago can be separated from other members of the genus by having palpomere 2 very long, its length more than half the length of palpomere 3 and sensorial pit with