In vitro study on the disinfectability of two split-septum needle-free connection devices using different disinfection procedures
In vitro-Untersuchungen zur Desinfizierbarkeit von zwei nadelfreien Split-Septum-Konnektionsventilen mit verschiedenen
Desinfektionsverfahren
Abstract
This in vitro study investigated the external disinfection of two needle- free connection devices (NFC) using Octeniderm®(spraying and wiping
Steffen Engelhart
1Martin Exner
1technique) vs. Descoderm®pads (wiping technique). The split-septum
Arne Simon
2membrane of the NFC was contaminated with >105CFUK. pneumoniae or S. epidermidis. The efficacy of the disinfection at 30 sec. exposure
time was controlled by taking a swab sample and by flushing the NFC 1 Institute for Hygiene and Public Health,
with sterile 0.9% sodium chloride solution. Disinfection with octenidine
dihydrochloride 0.1 g, 1-Propanol 30.0 g, and 2-Propanol 45.0 g in 100 g Universitätsklinikum Bonn, Germany
solution was highly effective (CFU reduction ≥4 log) against both microor- ganisms, whereas the use of 63.1 g 2-Propanol in 100 ml solution led
2 Pediatric Oncology and Hematology, Children's to residual contamination withS. epidermidis. Our investigation under-
lines that (i) in clinical practice disinfection of NFCs before use is man- University Hospital, Homburg, Saar, Germany
datory, and that (ii) details of disinfection technique are of utmost im- portance regarding their efficacy. Our investigation revealed no signifi- cant differences between both split-septum NFC types. Clinical studies are needed to confirm a possible superiority of disinfectants with long- lasting residual antimicrobial activity.
Keywords:needle-free connection devices (NFC), central venous catheter, hub disinfection, octenidine/propanol, 2-propanol
Zusammenfassung
In einer Reihe von in vitro-Versuchen wurde die Desinfizierbarkeit von zwei nadelfreien Split-Septum-Konnektionsventilen (NFC) mit einem Hautantiseptikum auf Basis von Octenidindihydrochlorid 0,1 g, 1-Propa- nol 30,0 g und 2-Propanol 45,0 g in 100 g Lösung mittels Sprüh- und Wischdesinfektion mit steriler Kompresse vs. Wischdesinfektion mit vorgetränkten Läppchen mit 63,1 g 2-Propanol in 100 ml Lösung unter- sucht. Dabei wurde die äußere Membran des NFC mit >105 KbE K. pneumoniae oder S. epidermidiskontaminiert. Die Überprüfung des Desinfektionserfolgs bei einer Einwirkungszeit von 30 Sek. erfolgte mittels Abstrich und Durchspülmethode.
Die Desinfektion mit dem Hautantiseptikum erwies sich in Bezug auf beide Bakterienspezies als hocheffektiv. Hingegen konnte nach der hier durchgeführten Methode der Desinfektion mit vorgetränkten Läppchen in einigen Versuchsreihen mitS. epidermidiskeine ausrei- chende Dekontamination beider NFC-Typen erreicht werden.
Die vorliegende Untersuchung unterstreicht, dass NFCs im klinischen Einsatz vor einer geplanten Konnektion immer der Desinfektion bedürfen und dass die Technik der Desinfektion von entscheidender Bedeutung ist. Eine klare Überlegenheit eines der beiden Konnektoren konnte nicht festgestellt werden. Ob Desinfektionsmittel mit einer Remanenzwirkung
tatsächlich überlegen sind, muss in weiterführenden Studien untersucht werden.
Schlüsselwörter:nadelfeie Konnektionsventile, zentraler Venenkatheter, Hub-Desinfektion, Octenidin/Propanol, 2-Propanol
Introduction
Besides full barrier precautions at insertion of central venous catheters (CVCs) and removal of unnecessary catheters as soon as possible [1], [2], maintenance care plays a major role in prevention of catheter bloodstream infections (BSI) originating from CVC surfaces or access ports [3].
There is a broad consensus that before any manipulation the catheter hub or other venous access sites have to be thoroughly disinfected [3], [4], [5], [6], [7], [8]. If frequent manipulations are necessary, a needle-free connection device (NFC) can be advantageous [9], as its disinfection is easier than disinfection of a three-way stopcock [10], [11], [12].
The manufacturers of NFCs are obliged to supply the user with information as to which disinfection methods and preparations are applicable for decontamination of the particular NFC-model’s access site during clinical use.
Some reports indicate that NFCs can even raise the risk of catheter-related bloodstream infections [13], in partic- ular positive pressure NFCs [14], [15], [16], [17], and in case of insufficient education and training concerning the correct use and disinfection of these devices [18], [19], [20].
In order to reach a high compliance (>95%) with the cor- rect use of NFCs [21], [22], the disinfection method must be practicable. A good example of a non-practical proce- dure is cited by Adams et al. [23]:
„…firmly applying individual swabs containing 70% (v/v) isopropyl alcohol (IPA) (Sterets; Seton Healthcare, Old- ham,UK) to the compression seal and rotating five times through 360°. The 70% (v/v) IPA was subsequently al- lowed to dry for 2 min.”
The time needed to complete the whole disinfection pro- cedure must not exceed 30 seconds which can reason- ably be used for additional hand disinfection.
Some experts recommend the use of a disinfectant combination including a long-lasting residual disinfectant activity, e.g. alcohol with chlorhexidine or octenidine, for hub disinfection [24], [25], [26] in analogy to skin anti- sepsis with chlorhexidine 2% / isopropanol 70% during insertion of CVCs [3].
This in vitro study investigates the disinfection of two split- septum NFC models (BD Q-Syte®und MicroClave®) using (1) an alcohol-based ready to use tissue (Descoderm® Pads) or (2) a propanol-octenidine containing disinfectant spray (Octeniderm®) under controlled conditions.
Materials and methods
NFC types
We investigated two different split-septum needle-free connection devices (NFC): BD Q-Syte®(Becton Dickinson, Heidelberg, Deutschland) [23], [10], and MicroCLAVE (NeoCare GmbH, Lüdenscheid, Deutschland) [27], [28], [29].
Test microorganisms and inoculation of the NFC membrane
The test microorganisms were Staphylococcus epidermidis (ATCC 12228) and Klebsiella pneumoniae subspecies pneumoniae (ATCC 13882). The test microor- ganisms were calibrated at a concentration of 108 CFU/mL, after dilution (1:10 with 0.9% sodium chloride solution) an aliquot of 10 µl was inoculated on the NFC membrane (inoculum >105CFU/NFC membrane) by pipette. Under the laminar air flow bench, the inoculum was dried over a period of 30 min.
Disinfectants
Descoderm Pads®(Dr. Schumacher GmbH, Melsungen, Germany) are single packaged pre-moistured pads, size:
6×3×8 cm, containing 63.1 g 2-Propanol in 100 ml solution as active component. The pads are commis- sioned for skin antisepsis before injection or puncture (exposure time 15 seconds). According to the manufac- turer, the pads can also be used to disinfect alcohol-re- sistant surfaces of medical devices (e.g., the rubber sur- face of infusion bottles) [30].
Octeniderm®(Schülke & Mayr GmbH, Norderstedt, Ger- many) was used as spray, containing octenidine dihydro- chloride 0.1 g, 1-propanol 30.0 g and 2-propanol 45.0 g in 100 g solution as active component. According to the manufacturer, the spray is commissioned for skin anti- sepsis before surgical operations as well as catheteriza- tion or puncture of blood vessels.
Both antiseptics are listed by the German Disinfectants Commission in the Association for Applied Hygiene.
Disinfection procedures
Descoderm®pad:The NFCs were placed on sterile gauze, and the connector was disinfected by wiping the connect- ing surface of the device with the pad one time clockwise with moderate digital pressure ensuring to reach the
complete surface. The exposure time of the disinfectant was 30 seconds (maximum, 40 seconds).
Octeniderm®:The NFCs were placed on sterile gauze, and the connecting surface of the device was disinfected with 4 puffs of the Octeniderm®sprayer. Then, the surface of the NFC was wiped with the Octeniderm® moistened sterile gauze with moderate digital pressure one time clockwise. After that, another two puffs of the Octeniderm® spray were applied. Corresponding to the Descoderm® pad procedure, the exposure time of the disinfectant was at least 30 seconds (maximum, 40 seconds). The doubled positive controls remained without disinfection.
Laboratory analysis
Contact samples of the connector membrane:The outer surface of the membrane including the split was swabbed with a sterile 0.9% NaCl moistened swab (Greiner, Ger- many; article number 420180); the sample was directly transferred to Columbia 5% sheep blood (SB) and cultured for 24 h at 36°C. For the positive control the outer sur- face of the membrane including the split was swabbed with a sterile 0.9% NaCl moistened swab; the tip of the swab was vortexed in a tube containing 4.5 ml 0.9% NaCl and processed for quantitative culture using standard dilution techniques (Columbia 5% SB; 24 h / 36°C) Flushing samples:A sample of 100 ml sterile NaCl 0.9%
saline flush for each device was collected in a separate sterile membrane filter funnel unit; the membrane filter was then transferred to the surface of a Columbia 5%
plate (incubated for 24 h / 36°C). For the positive control a sample of 100 ml sterile NaCl 0.9% saline flush for each device was collected in a separate sterile glass and processed for quantitative culture using standard dilution techniques (Columbia 5% SB; 24 h / 36°C).
For any combination of the two connectors, disinfection procedures, sample types, and test microorganisms, a total of 8 separate samples and corresponding 2 positive controls each were conducted. Reduction factors were calculated as the differences between the single results from the arithmetic means (log) and the corresponding positive controls (log), respectively.
Results
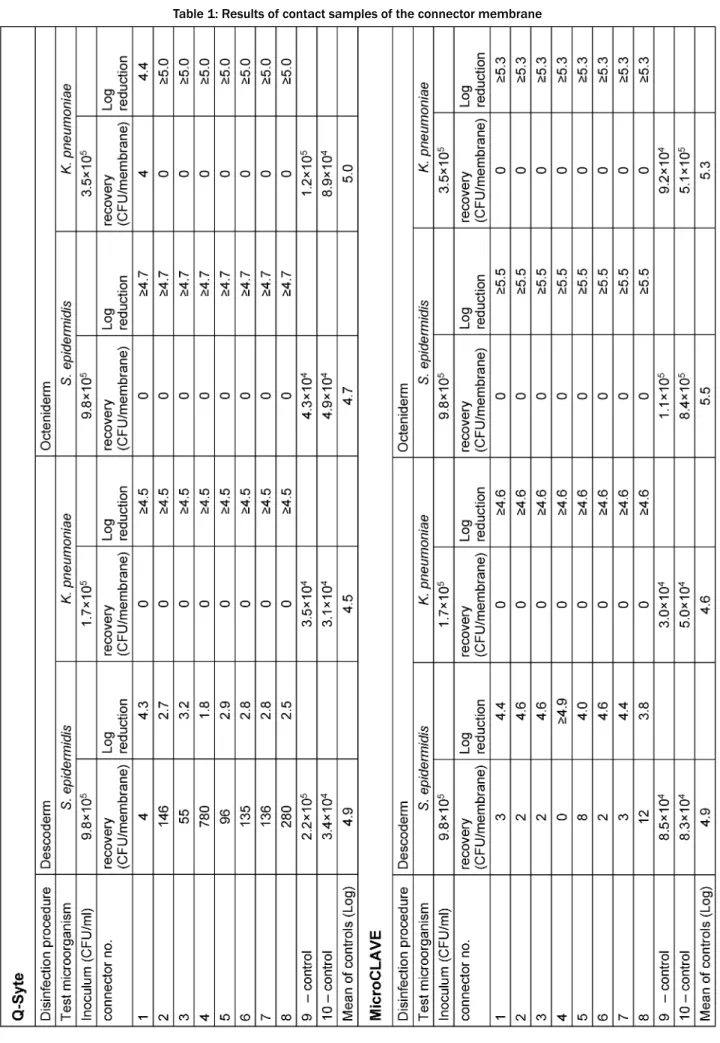
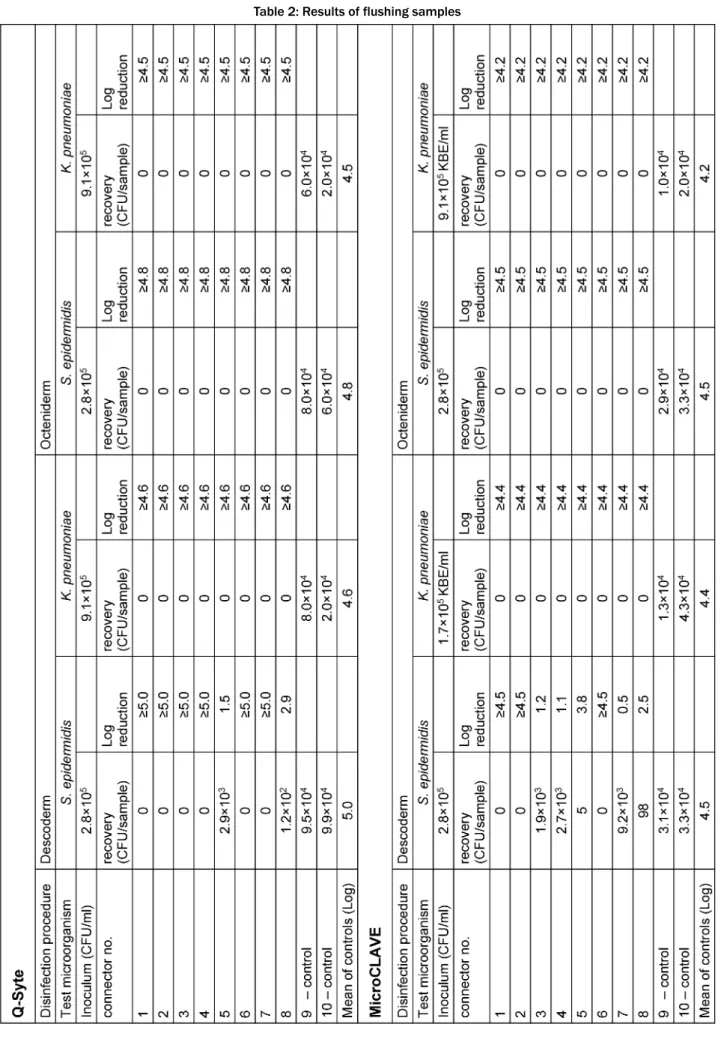
Table 1 presents the results of the contact (swab) samples, Table 2 those of the flushing samples. Regard- ing both sample types, we found good recovery (nearly throughout <1 log difference compared with the primary inoculum) in the doubled control samples.
After disinfection with Octeniderm®both NFC types re- vealed no residual contamination in all contact and flushing samples (both test microorganisms with >4 log reduction factor).
In contrast, disinfection with Descoderm®pads yielded discordant results: contamination withK. pneumoniawas completely removed from both NFC types (>4 log reduc- tion factor), but contamination withS. epidermidiswas
not completely eliminated. 6 of 8 contact samples from BD Q-Syte®as well as 2 of 8 flushing samples yielded
<3 log reduction factor. The corresponding results for MicroCLAVE® were 1 of 8 contact samples and 4 of 8 flushing samples (resulting reduction factors only between 0.5 to 2.5 log).
Discussion
Both split septum NFC models could successfully be dis- infected using the Octeniderm®procedure as described above within 30 seconds. Even with a high artificial in- oculum (>105CFU), creating a worst-case scenario com- pared to bacterial contamination of NFCs in clinical practice [31], no bacterial pathogens (Klebsiella pneumo- niae orS. epidermidis) could be detected on the mem- brane or in the infusate after disinfection. The exposure time of 30 seconds can favorably be used to conduct hand disinfection before the NFC is accessed.
In contrast, the Descoderm®pad procedure did not show sufficient efficacy regarding inactivation ofS. epidermidis in several samples (<4 log reduction and/or detection of microorganisms in flushing samples). S. epidermidisis one of the most frequently detected Gram-positive mi- croorganisms detected on hands and hand contact sur- faces/fomites and one of the leading pathogens in cath- eter-related bloodstream infections [3].
Apparently, our results are in contradiction with results from other authors on the efficacy of Descoderm®pads [30], [10]. In addition, the manufacturer of the BD Q-Site® NFC recommends wiping the membrane of the NFC for 60 seconds with a moistured pad containing propanol as active component (e.g. Descoderm®; pers. communication with Becton Dickinson, Oct. 07/2015). For clarification of this discrepancy, the disinfection procedure has to be discussed in detail. Trautmann et al. describe a disinfec- tion procedure of a different NFC model (Bionecteur®, Vygon GmbH, Aachen) as “vigorous circular wiping disin- fection of the membrane” and subsequent waiting (30 seconds) until the membrane has fully dried [30].
Thus, our disinfection procedure implies a less intensive mechanic component (just a single wiping course at moderate pressure), on the other hand a shorter exposure time (30 sec vs. 60 sec) compared to the manufacturer’s recommendation.
The different outcomes of the Descoderm®pad resp. the Octeniderm®procedure must also be discussed: several authors described the long-lasting residual disinfectant activity of octenidine in vitro [32] and in vivo when applied on the skin and mucous membranes [33], [34], [35].
However, it remains to be elucidated whether this effect translates into superior clinical efficacy in terms of BSI prevention. At least, some non-randomized clinical cohort studies [24], [25], [26], including a study on a bundle of preventive interventions from a German pediatric oncol- ogy department [36] seem to argue for a substantial benefit. It can also not fully be ruled out that octenidine residues could have had an inhibitory effect on the cultur-
Table 1: Results of contact samples of the connector membrane
Table 2: Results of flushing samples
ability of regained microorganisms in vitro, however, in the case of the flushing samples this seems improbable due to the high dilution factor and no inhibition zone of the flushing sample in a disc diffusion assay. Also, the different tissue characteristics of the pads versus the gauze could have played a minor role (e.g., higher surface adhesion of the gauze).
In summary, we conclude that (i) the clinical use of NFCs mandatorily requires a previous disinfection before any connection, and (ii) the detailed technique of the disinfec- tion procedure is of utmost importance and thus should be thoroughly defined, educated and trained.
We found no clear superiority of one of the NFC models examined in our study, at best a lower rate of positive flushing samples in case of the BD Q-Syte®. Further studies are needed regarding a possible superiority of disinfectant combinations revealing a long-lasting residual disinfectant activity in this clinical context.
Notes
Competing interests
The study was part of a project “Intervention bundles on prevention of catheter-related bloodstream infections in pediatric Oncology”, which was funded by the companies Schülke & Mayr GmbH, Norderstedt, Germany, and Becton Dickinson, Heidelberg, Germany.
In advance to the study, an open discussion concerning the scientific (experimental) state-of-the-art took place including experts from both companies. None of the companies had any influence on study design, realization and evaluation of the study, or on this publication.
References
1. Berenholtz SM, Lubomski LH, Weeks K, Goeschel CA, Marsteller JA, Pham JC, Sawyer MD, Thompson DA, Winters BD, Cosgrove SE, Yang T, Louis TA, Meyer Lucas B, George CT, Watson SR, Albert-Lesher MI, St Andre JR, Combes JR, Bohr D, Hines SC, Battles JB, Pronovost PJ. Eliminating central line-associated bloodstream infections: a national patient safety imperative.
Infect Control Hosp Epidemiol. 2014;35(1):56-62. DOI:
10.1086/674384
2. Pronovost PJ, Goeschel CA, Colantuoni E, Watson S, Lubomski LH, Berenholtz SM, Thompson DA, Sinopoli DJ, Cosgrove S, Sexton JB, Marsteller JA, Hyzy RC, Welsh R, Posa P, Schumacher K, Needham D. Sustaining reductions in catheter related bloodstream infections in Michigan intensive care units:
observational study. BMJ. 2010;340:c309. DOI:
10.1136/bmj.c309
3. Marschall J, Mermel LA, Fakih M, Hadaway L, Kallen A, O'Grady NP, Pettis AM, Rupp ME, Sandora T, Maragakis LL, Yokoe DS.
Strategies to prevent central line-associated bloodstream infections in acute care hospitals: 2014 update. Infect Control Hosp Epidemiol. 2014;35(7):753-71. DOI: 10.1086/676533 4. O'Grady NP, Alexander M, Burns LA, Dellinger EP, Garland J,
Heard SO, Lipsett PA, Masur H, Mermel LA, Pearson ML, Raad, II, Randolph AG, Rupp ME, Saint S. Guidelines for the prevention of intravascular catheter-related infections. Am J Infect Control.
2011;39(4 Suppl 1):S1-34. DOI: 10.1016/j.ajic.2011.01.003
5. Rupp ME, Yu S, Huerta T, Cavalieri RJ, Alter R, Fey PD, Van Schooneveld T, Anderson JR. Adequate disinfection of a split- septum needleless intravascular connector with a 5-second alcohol scrub. Infect Control Hosp Epidemiol. 2012;33(7):661- 5. DOI: 10.1086/666337
6. Wright MO, Tropp J, Schora DM, Dillon-Grant M, Peterson K, Boehm S, Robicsek A, Peterson LR. Continuous passive disinfection of catheter hubs prevents contamination and bloodstream infection. Am J Infect Control. 2013;41(1):33-8.
DOI: 10.1016/j.ajic.2012.05.030
7. Simmons S, Bryson C, Porter S. "Scrub the hub": cleaning duration and reduction in bacterial load on central venous catheters. Crit Care Nurs Q. 2011;34(1):31-5. DOI:
10.1097/CNQ.0b013e3182048073
8. Lockman JL, Heitmiller ES, Ascenzi JA, Berkowitz I. Scrub the hub! Catheter needleless port decontamination. Anesthesiology.
2011;114(4):958. DOI: 10.1097/ALN.0b013e3182054bd1 9. Simon A, Trautmann M. Nadelfreie Konnektionsventile und
Blutstrominfektionen - Kommentar aus klinischer Sicht [Needleless connection valves - commentary from a clinical perspective]. Dtsch Med Wochenschr. 2008;133(5):206-8. DOI:
10.1055/s-2008-1017499
10. Pohl F, Hartmann W, Holzmann T, Gensicke S, Kolbl O, Hautmann MG. Risk of infection due to medical interventions via central venous catheters or implantable venous access port systems at the middle port of a three-way cock: luer lock cap vs. luer access split septum system (Q-Syte). BMC Infect Dis. 2014;14:41. DOI:
10.1186/1471-2334-14-41
11. Trautmann M, Moosbauer S, Schmitz FJ, Lepper PM.
Experimental study on the safety of a new connecting device.
Am J Infect Control. 2004;32(5):296-300. DOI:
10.1016/j.ajic.2004.03.002
12. Oto J, Nishimura M, Morimatsu H, Katayama H, Onodera M, Takahashi H, Takezawa J. Comparison of contamination between conventional three-way stopcock and needleless injection device:
a randomized controlled trial. Med Sci Monit.
2007;13(10):CR417-21.
13. McKee C, Berkowitz I, Cosgrove SE, Bradley K, Beers C, Perl TM, Winner L, Pronovost PJ, Miller MR. Reduction of catheter- associated bloodstream infections in pediatric patients:
experimentation and reality. Pediatr Crit Care Med. 2008;9(1):40- 6. DOI: 10.1097/01.PCC.0000299821.46193.A3
14. Maragakis LL, Bradley KL, Song X, Beers C, Miller MR, Cosgrove SE, Perl TM. Increased catheter-related bloodstream infection rates after the introduction of a new mechanical valve intravenous access port. Infect Control Hosp Epidemiol.
2006;27(1):67-70. DOI: 10.1086/499166
15. Salgado CD, Chinnes L, Paczesny TH, Cantey JR. Increased rate of catheter-related bloodstream infection associated with use of a needleless mechanical valve device at a long-term acute care hospital. Infect Control Hosp Epidemiol. 2007;28(6):684- 8. DOI: 10.1086/516800
16. Btaiche IF, Kovacevich DS, Khalidi N, Papke LF. The effects of needleless connectors on catheter-related bloodstream infections. Am J Infect Control. 2011;39(4):277-83. DOI:
10.1016/j.ajic.2010.07.011
17. Jarvis WR, Murphy C, Hall KK, Fogle PJ, Karchmer TB, Harrington G, Salgado C, Giannetta ET, Cameron C, Sherertz RJ. Health care- associated bloodstream infections associated with negative- or positive-pressure or displacement mechanical valve needleless connectors. Clin Infect Dis. 2009;49(12):1821-7. DOI:
10.1086/648418
18. Rupp ME, Sholtz LA, Jourdan DR, Marion ND, Tyner LK, Fey PD, Iwen PC, Anderson JR. Outbreak of bloodstream infection temporally associated with the use of an intravascular needleless valve. Clin Infect Dis. 2007;44(11):1408-14. DOI:
10.1086/517538
19. Yebenes JC, Serra-Prat M. Clinical use of disinfectable needle- free connectors. Am J Infect Control. 2008;36(10):S175. e171- 4. DOI: 10.1016/j.ajic.2008.10.013
20. Kellerman S, Shay DK, Howard J, Goes C, Feusner J, Rosenberg J, Vugia DJ, Jarvis WR. Bloodstream infections in home infusion patients: the influence of race and needleless intravascular access devices. J Pediatr. 1996;129(5):711-7. DOI:
10.1016/S0022-3476(96)70154-3
21. Smith JS, Kirksey KM, Becker H, Brown A. Autonomy and Self- efficacy as Influencing Factors in Nurses' Behavioral Intention to Disinfect Needleless Intravenous Systems. J Infus Nurs.
2011;34(3):193-200. DOI: 10.1097/NAN.0b013e31821478e7 22. Resar RK. Making noncatastrophic health care processes reliable:
Learning to walk before running in creating high-reliability organizations. Health Serv Res. 2006;41(4 Pt 2):1677-89. DOI:
10.1111/j.1475-6773.2006.00571.x
23. Adams D, Karpanen T, Worthington T, Lambert P, Elliott TS.
Infection risk associated with a closed luer access device. J Hosp Infect. 2006;62(3):353-7. DOI: 10.1016/j.jhin.2005.09.016 24. Soothill JS, Bravery K, Ho A, Macqueen S, Collins J, Lock P. A fall
in bloodstream infections followed a change to 2% chlorhexidine in 70% isopropanol for catheter connection antisepsis: a pediatric single center before/after study on a hemopoietic stem cell transplant ward. Am J Infect Control. 2009;37(8):626-30. DOI:
10.1016/j.ajic.2009.03.014
25. Horvath B, Norville R, Lee D, Hyde A, Gregurich M, Hockenberry M. Reducing central venous catheter-related bloodstream infections in children with cancer. Oncol Nurs Forum. 2009;36 (2):232-8. DOI: 10.1188/09.ONF.232-238
26. Hong H, Morrow DF, Sandora TJ, Priebe GP. Disinfection of needleless connectors with chlorhexidine-alcohol provides long- lasting residual disinfectant activity. Am J Infect Control.
2013;41(8):e77-9. DOI: 10.1016/j.ajic.2012.10.018
27. Chernecky C, Waller J. Comparative evaluation of five needleless intravenous connectors. J Adv Nurs. 2011;67(7):1601-3. DOI:
10.1111/j.1365-2648.2010.05598.x
28. Yebenes JC, Delgado M, Sauca G, Serra-Prat M, Solsona M, Almirall J, Capdevila JA, Balanzo X. Efficacy of three different valve systems of needle-free closed connectors in avoiding access of microorganisms to endovascular catheters after incorrect handling. Crit Care Med. 2008;36(9):2558-61. DOI:
10.1097/CCM.0b013e318183effb
29. Bouza E, Munoz P, Lopez-Rodriguez J, Jesus Perez M, Rincon C, Martin Rabadan P, Sanchez C, Bastida E. A needleless closed system device (CLAVE) protects from intravascular catheter tip and hub colonization: a prospective randomized study. J Hosp Infect. 2003;54(4):279-87. DOI: 10.1016/S0195-
6701(03)00136-1
30. Trautmann M, Kreutzberger M, Bobic R, Regnath T. Disinfection of a needleless connector with alcohol-based disinfectant wipes – an experimental study. Hyg Med. 2012;37(9):354-9.
31. Longtin Y, Schneider A, Tschopp C, Renzi G, Gayet-Ageron A, Schrenzel J, Pittet D. Contamination of stethoscopes and physicians' hands after a physical examination. Mayo Clin Proc.
2014;89(3):291-9. DOI: 10.1016/j.mayocp.2013.11.016 32. Müller G, Langer J, Siebert J, Kramer A. Residual antimicrobial
effect of chlorhexidine digluconate and octenidine dihydrochloride on reconstructed human epidermis. Skin Pharmacol Physiol.
2014;27(1):1-8. DOI: 10.1159/000350172
33. Dettenkofer M, Jonas D, Wiechmann C, Rossner R, Frank U, Zentner J, Daschner FD. Effect of skin disinfection with octenidine dihydrochloride on insertion site colonization of intravascular catheters. Infection. 2002;30(5):282-5. DOI: 10.1007/s15010- 002-2182-2
34. Dettenkofer M, Wilson C, Gratwohl A, Schmoor C, Bertz H, Frei R, Heim D, Luft D, Schulz S, Widmer AF. Skin disinfection with octenidine dihydrochloride for central venous catheter site care:
a double-blind, randomized, controlled trial. Clin Microbiol Infect.
2010;16(6):600-6. DOI: 10.1111/j.1469-0691.2009.02917.x 35. Hubner NO, Siebert J, Kramer A. Octenidine dihydrochloride, a modern antiseptic for skin, mucous membranes and wounds.
Skin Pharmacol Physiol. 2010;23(5):244-58. DOI:
10.1159/000314699
36. Furtwängler R, Laux C, Graf N, Simon A. Impact of a modified Broviac maintenance care bundle on bloodstream infections in paediatric cancer patients. GMS Hyg Infect Control.
2015;10:Doc15. DOI: 10.3205/dgkh000258
Corresponding author:
Prof. Dr. med. Steffen Engelhart
Universitätsklinikum Bonn, Institut für Hygiene und Öffentliche Gesundheit, Sigmund-Freud-Str. 25, Gebäude 63, 53127 Bonn, Germany, Phone: 0228-287 14434, Fax: 0228-287 15645
steffen.engelhart@ukb.uni-bonn.de
Please cite as
Engelhart S, Exner M, Simon A. In vitro study on the disinfectability of two split-septum needle-free connection devices using different disinfection procedures. GMS Hyg Infect Control. 2015;10:Doc17.
DOI: 10.3205/dgkh000260, URN: urn:nbn:de:0183-dgkh0002600
This article is freely available from
http://www.egms.de/en/journals/dgkh/2015-10/dgkh000260.shtml Published:2015-12-09
Copyright
©2015 Engelhart et al. This is an Open Access article distributed under the terms of the Creative Commons Attribution 4.0 License. See license information at http://creativecommons.org/licenses/by/4.0/.