Research Collection
Working Paper
Nano-3D-printed Photochromic Objects
Author(s):
Ulrich, Sebastian; Wang, Xiaopu; Rottmar, Markus; Rossi, Rene M.; Nelson, Bradley; Bruns, Nico; Müller, Ralph; Maniura-Weber, Katharina; Qin, Xiao-Hua; Boesel, Luciano F.
Publication Date:
2020-08-31 Permanent Link:
https://doi.org/10.3929/ethz-b-000464517
Originally published in:
ChemRxiv , http://doi.org/10.26434/chemrxiv.12896501.v1
Rights / License:
Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International
This page was generated automatically upon download from the ETH Zurich Research Collection. For more information please consult the Terms of use.
ETH Library
Nano-3D-printed Photochromic Objects
Sebastian Ulrich, Xiaopu Wang, Markus Rottmar, René M. Rossi, Bradley J. Nelson, Nico Bruns, Ralph Müller, Katharina Maniura-Weber, Xiao-Hua Qin, Luciano F. Boesel
Submitted date: 31/08/2020 • Posted date: 01/09/2020 Licence: CC BY-NC-ND 4.0
Citation information: Ulrich, Sebastian; Wang, Xiaopu; Rottmar, Markus; Rossi, René M.; Nelson, Bradley J.;
Bruns, Nico; et al. (2020): Nano-3D-printed Photochromic Objects. ChemRxiv. Preprint.
https://doi.org/10.26434/chemrxiv.12896501.v1
A new class of photoresist is described for direct laser writing of photoswitchable 3D microstructures. The material comprising off-stoichiometric thiol-ene photo-clickable resins enables rapid two-photon laser
processing of highly complex structures and facile post-modification with photoswitches. The microstructures were functionalized with a series of donor-acceptor Stenhouse adducts (DASAs) photoswitches with different excitation wavelength. The versatility of thiol–ene photo-click reaction enabled fine-tuning of the network structure and physical properties as well as the type and concentration of DASA photoswitches. When
exposed to visible light, these microstructures exhibit excellent photo-responsiveness and undergo reversible color-changing via photoisomerization of DASA moieties. We describe that the weak fluorescence of DASAs can be used as a reporter of photoswitching, color changes, and thermal recovery, allowing the reading of DASA-containing sub-micrometric structures in 3D. This work delivers a new approach for custom
microfabrication of 3D photochromic objects with molecularly engineered color and responsiveness.
File list (2)
download file view on ChemRxiv
Ulrich_2020.pdf (0.97 MiB)
download file view on ChemRxiv
Ulrich_2020 SI.pdf (2.06 MiB)
1 3D-printed Photochromism
Nano-3D-printed Photochromic Objects
Sebastian Ulrich1,3, Xiaopu Wang2,6, Markus Rottmar4, René Michel Rossi1,Bradley J. Nelson2, Nico Bruns3, Ralph Müller5, Katharina Maniura-Weber4, Xiao-Hua Qin4,5,* and Luciano Fernandes Boesel1
1 Empa, Swiss Federal Laboratories for Materials Science and Technology, Laboratory for Biomimetic Membranes and Textiles
Lerchenfeldstrasse 5, 9014 St. Gallen, Switzerland
2 Institute of Robotics and Intelligent Systems ETH Zurich
Tannenstrasse 3, 8092 Zurich, Switzerland
3 Adolphe Merkle Institute University of Fribourg
Chemin des Verdiers 4, 1700 Fribourg, Switzerland
4 Empa, Swiss Federal Laboratories for Materials Science and Technology Biointerfaces
Lerchenfeldstrasse 5, 9014 St. Gallen, Switzerland
5 Institute for Biomechanics ETH Zurich
Leopold-Ruzicka-Weg 4, 8093 Zurich, Switzerland
6 Shenzhen Institute of Artificial Intelligence and Robotics for Society The Chinese University of Hong Kong, Shenzhen
518172 Shenzhen, China E-mail: qinx@ethz.ch
Keywords: Two-photon polymerization, nanoscale 3D-printing, thiol-ene reaction, donor- acceptor stenhouse adducts, photochromism
2
Abstract
A new class of photoresist is described for direct laser writing of photoswitchable 3D microstructures. The material comprising off-stoichiometric thiol-ene photo-clickable resins enables rapid two-photon laser processing of highly complex structures and facile post- modification with photoswitches. The microstructures were functionalized with a series of donor-acceptor Stenhouse adducts (DASAs) photoswitches with different excitation wavelength. The versatility of thiol–ene photo-click reaction enabled fine-tuning of the network structure and physical properties as well as the type and concentration of DASA photoswitches.
When exposed to visible light, these microstructures exhibit excellent photo-responsiveness and undergo reversible color-changing via photoisomerization of DASA moieties. We describe that the weak fluorescence of DASAs can be used as a reporter of photoswitching, color changes, and thermal recovery, allowing the reading of DASA-containing sub-micrometric structures in 3D. This work delivers a new approach for custom microfabrication of 3D photochromic objects with molecularly engineered color and responsiveness.
3 Introduction
Stimuli-responsive materials based on organic photoswitches can reversibly change color and other properties when a light stimulus is applied, enabling their remote control with high spatial and temporal precision.[1-3] The controlled change of properties upon light irradiation has been exploited for a broad range of applications ranging from photochromic ophthalmic lenses to optical data storage, optical switches, phase shifters, sensors, drug delivery and actuators for soft robotics.[4-8] Most established systems, however, require UV light, which provides a very limited penetration depth into many materials, including skin, and is often detrimental to their structure.[9] These limitations have boosted the search for visible light- responsive photoswitches. One the most remarkable developments in this field was the discovery of donor-acceptor Stenhouse adducts (DASAs), a new class of visible light- responsive photoswitches with negative photochromism.[10-12] With a rapidly increasing knowledge on their structure-property relationships and their subsequent optimization, 2nd and 3rd generations of DASAs have overcome initial limitations, providing now access to the whole range of the visible spectrum with excellent photoswitching properties both in solution and in polymer matrices.[13-15] In materials science, DASA have already been employed in multiple applications from sensors to wavelength-specific photopatterning and visible light responsive enzyme nanoreactors.[16-26] In such multi-addressable materials, DASAs serve as molecular logic gates that only require different visible light sources to control the different states.[27, 28]
To date, however, DASAs have been studied mostly in two-dimensional (2D) photoswitchable substrates such as polymer films and modified surfaces. Combining the wavelength-specific activation of DASA with complex-shaped objects could allow the fabrication of materials with sophisticated control both on the functional and the structural levels. Yet, approaches to fabricate customized 3D photochromic objects based on DASAs have to be established first.
Recent advances in 3D-printing technologies have witnessed their burgeoning applications in digital fabrication, microelectronics and biomedicine.[29-31] One of the most precise 3D-
4
printing technologies is two-photon photolithography (2PP),[32, 33] which relies on localized curing of liquid ink materials upon non-linear two-photon photoinitiation triggered by an ultrafast near-infrared laser beam. Since most polymeric materials are transparent at 800 nm, one can easily build custom 3D micro-objects in accordance with computer-aided design,[32]
or even control material properties in space and time at submicron-level resolution.[33, 34]
Radical-mediated thiol-ene click reactions are particularly suitable for photocrosslinking due to their high reactivity and selectivity through the fast initiation with radical photoinitiators and subsequent chain-transfer reactions.[35, 36] While 2PP has been used to fabricate optical devices such as waveguides,[37] so far there are no reports on 3D-printed photochromic micro- objects that allow localized dynamic tuning of color on demand by visible light.
Herein, we describe a novel approach to create visible light-responsive photochromic 3D micro-objects by interfacing ink formulations containing DASAs with nanoscale 3D-printing technique based on 2PP. We firstly designed and formulated a highly efficient thiol-ene photo- clickable ink system that consists of multifunctional thiols/enes and an ene-terminated DASA precursor. This ink system is modular and its robustness for thiol-ene photocrosslinking enables ultrafast laser microfabrication of complex 3D objects at high writing speeds up to 50 mm∙s-1. The microstructures can be functionalized with a series of DASA photoswitches with wavelength-specific switching properties in a modular manner. By scanning a focused green laser inside the functionalized microstructure, we demonstrate photodynamic color-tuning of DASA-containing polymer networks at high spatial resolution as well as their reversible photoswitching. We demonstrate that the weak fluorescence of DASAs can be used as a reporter of photoswitching and color changes in sub-micrometric structures, for which standard techniques (e.g., UV-Vis spectrometry) are difficult to apply, either because the structures are too small or because they require 3D-reading capability.
Results
Synthetic Concept and Network Design
5
We designed a modular ink system to print visible light-responsive photochromic DASA- networks by radical thiol-ene photopolymerization. The ink system is composed of stoichiometric mixtures of pentaerythritol tetra(3-mercaptopropionate) (PETMP, 4Thiol), triallyl-1,3,5-triazine-2,4,6-trione (TATATO, 3Ene), and an ene-terminated DASA precursor (4-methoxy-N-(pent-4-en-1-yl)aniline, EnePre) from a 2-step synthesis (Figure 1A). Using diphenyl(2,4,6-trimethylbenzoyl)phosphine oxide (TPO) as the photoinitiator, an off- stoichiometric thiol-ene photocrosslinking was performed upon exposure to either UV light for one-photon curing or near-infrared laser pulses for 2PP. After color developing these networks in a solution of activated furan adducts (FAs) where the DASAs are formed by reaction with the aromatic amine, photochromic DASA polymer networks were generated (Figure 1A). Three different FA based on Meldrum’s acid (Meld), N-substituted barbituric acid (Barb) or a pyrazolone (Pyra) were employed. While the first two FA have been employed already in the first reports of DASA photoswitches,[15] Pyra was the first of a new set of FA, pushing the DASA absorbance towards higher wavelengths.[22] The precursor-based approach offers several advantages: First, it minimizes the risk of photodamage to DASAs during photopolymerization but keeps the capacity to fabricate 3D micro-objects composed of DASA- networks through nanoscale 3D-printing via 2PP. Second, simple variation of the FA provides access to multiple DASAs with different colors and photoswitching properties from the same precursor network. With the rapid development of new FAs, the approach can be expanded to include novel DASA types with different or improved properties and functionalities. Finally, by varying the stoichiometry of thiol-ene monomers the network structure, composition, and mechanical properties can be tuned to optimize their performance as a photochromic matrix.
To investigate the thiol-ene click photopolymerization and the resulting network properties, we synthesized a number of networks with different compositions by tuning the stoichiometry of the monomers (see Table 1 for samples and their nomenclature). After development of a
6
network, for example, 2:1-1 with the Meld towards the Meldrum’s acid-based DASAs, it was termed 2:1-1MD. Accordingly, it was termed 2:1-1BD for Barb and 2:1-1PD for Pyra, with MD, BD, and PD designating the Meld-, Barb-, and Pyra-based DASAs (Figure 1B). Due to the intense color of DASAs, with molar extinction coefficients of the open form in the range of
∼105 M-1∙cm-1,[10] only small amounts of DASAs are typically needed to provide strong coloration.
Network Structure and Mechanical Properties
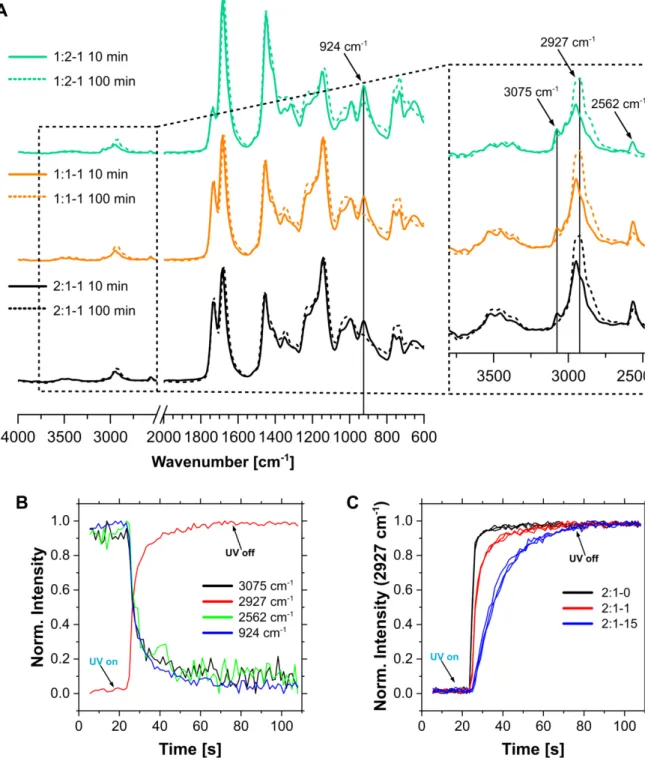
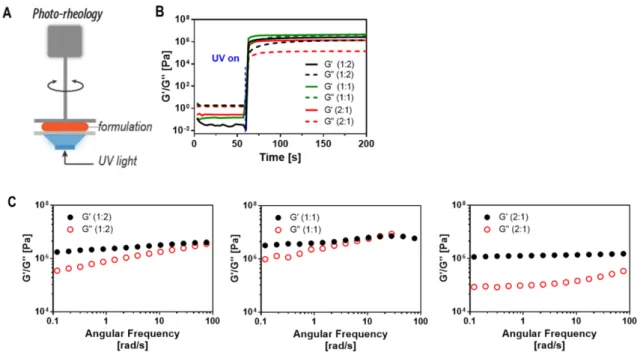
Figure 1. (A) Fabrication of DASA-networks via radical thiol-ene click photopolymerization (1PP or 2PP) of three monomers (4Thiol, 3Ene, and EnePre) for modular color development with three activated furan adducts (FAs): Meld, Barb, or Pyra. (B) Structures of the formed DASAs based on Meld (MD), Barb (BD) and Pyra (PD) FAs, respectively. (C) UV-vis spectra of the TPO initiator and EnePre: single-photon absorption at 390 nm, corresponding to half of the laser center wavelength (780 nm) for two-photon photolithography is marked with a blue line. (D) ATR-FTIR spectra of precursor networks with different network compositions and one DASA-network.
The spectra show key features of the network molecular structures, derived from the precursor monomers (compare with Figure S2). (E) In situ monitoring of thiol-ene photopolymerization kinetics of networks with different compositions using ATR-FTIR. (F) The effect of thiol-ene ratio on matrix mechanics - complex modulus (G*) as determined by photo-rheology.
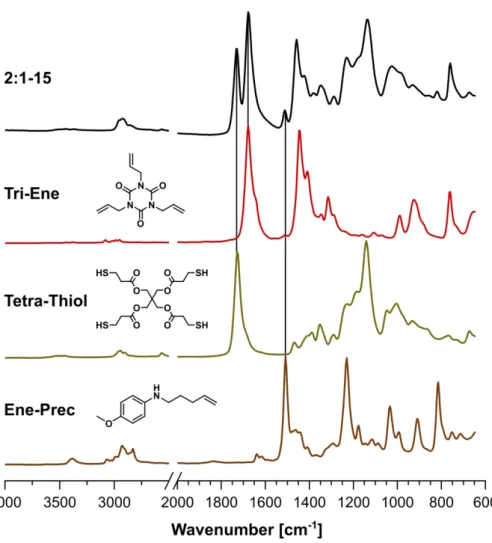
The chemical compositions of our ink system were investigated by attenuated total reflectance Fourier-transformed infrared (ATR-FTIR) spectroscopy. FTIR spectra of the different precursor networks are presented in Figure 1D and the spectra of both monomers and
7
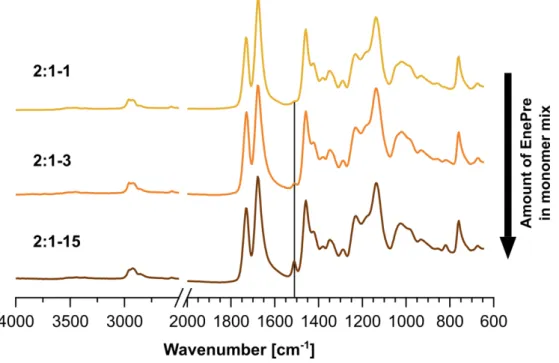
EnePre are shown for comparison in Figure S1. The different compositions of the networks are mirrored by the relative intensities of the ester peak of 4Thiol (1730 cm-1) and the imide peak of 3Ene (1674 cm-1). An excess of ene groups in the monomer mixture (1:2-1) resulted in the complete disappearance of the thiol signal at 2562 cm-1 while an excess of thiol groups (2:1-1) resulted in the disappearance of the ene signal at 924 cm-1. The disappearance of both signals for an equal ratio (1:1-1) indicates a high conversion of the functional groups during thiol-ene photopolymerization. A high degree of monomer incorporation into the network is supported by the small difference between unwashed precursor networks and ones that were washed in organic solvent (Figure S4). The successful incorporation of EnePre was confirmed by the appearance of a characteristic peak of the aromatic amine at 1512 cm-1. Higher concentrations, for example for 2:1-15 and for 2:1-3 were incorporated into the networks as well, demonstrating good control over the degree of network functionalization (Figure 1D, bottom, and SI5).
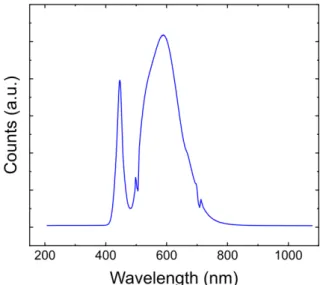
Next, we investigated the kinetics of thiol-ene photopolymerization (1PP) by in situ ATR- FTIR.[38] The peak at 2927 cm-1 corresponds to the formation of a methylene group during the polymerization. The monomer mixture without EnePre reacted extremely fast, being cured after an exposure to UV light for 3 s, indicating the excellent efficiency of radical-mediated thiol- ene click reaction.(Figure 1E). The presence of EnePre retarded the polymerization in a concentration dependent manner, though it still occurred within few seconds for the practically relevant formulations with 0.01 eq. of EnePre. Notably, the stoichiometry of the monomer components did not have any observable effect on the polymerization kinetics. The retarding effect of EnePre can be explained by the overlap of its absorption spectrum with the one of TPO initiator in the UV region (Figure 1C). However, such an overlap does not exist during the two- photon absorption of TPO at 780 nm. Therefore, 2PP can be expected to occur without interference. Comparison of full spectra from the early and late stages of the polymerization further confirmed that functional groups are consumed during the polymerization process and not removed later during the washing step (Figure S6A). Monitoring at different wavelengths
8
corresponding to the Cvinyl-H bond (3075 cm-1), the thiol group (2562 cm-1), or the vinyl group (924 cm-1) gave the same result (Figure S6B). Furthermore, independent repetitions of the experiments confirmed excellent reproducibility (Figure S6C).
The physical properties of a polymer matrix, in particular its stiffness/softness and the amount of polymer free volume can impede or allow the required dynamic motion on the molecular level during photoswitching.[39, 40] Therefore, we utilized in situ photorheology (Figure S7) to assess the mechanical properties and photoreactivity of the resulting networks.
Soft polymer matrices from low-Tg polymers generally provide the best conditions for the photoswitching of photochromic compounds with large changes in molecular shape.[41]
Previous reports on DASAs that isomerize from a linear extended form into a cyclic and compact form have emphasized the importance of such matrix properties, with only soft low- Tg matrices enabling good performances.[15, 25]
The measurements confirmed the fast polymerization kinetics. All changes occurred within 3 s, which is the time resolution of the photorheology setup. The speed of the network formation as determined by photorheology appears to be even faster than in the FTIR experiments. The difference is explained by the use of multi-functional monomers in a step-growth polymerization that results in an early gel point, i.e. network formation when a portion of the functional groups is still reacting, a behavior already observed by us for other multifunctional polymerizations.[42] The mechanical properties of the networks were found to strongly depend on the ratio of the monomer components (Figure 1F, left panel): A much softer network was obtained from the 2:1-1 composition in comparison to the networks from 1:2-1 and 1:1-1 compositions. Both the molecular structure of the monomers and the crosslinking density of the networks determine their mechanical properties. The more rigid structure of 1:1-1 indicates that the crosslinking density plays a major role. Moreover, the more rigid molecular structure of the cyclic 3Ene monomer appears to result in a more rigid network (compare 1:2-1 vs. 2:1-1). With the contributions of both lower crosslinking density and lower amount of rigid monomers, the
9
loss factor of 2:1-1 networks (Figure 1F, right panel) is about 0.1, which is much lower when compared to the other two networks. The lower the loss factor, the weaker the damping properties of the networks.
Furthermore, we employed dynamic rheology to assess the deformability of the networks.
Dynamic frequency sweep experiments (Figure S7C) show a clear difference in network structure. For the stiffer networks, the storage modulus (G’) and loss modulus (G’’) are cross- sectioned at high angular frequencies. In contrast, the G’ of soft networks (2:1-1) remain constantly higher than G’’ in the whole range, indicating a distinct difference in microstructure compared to the other two networks.
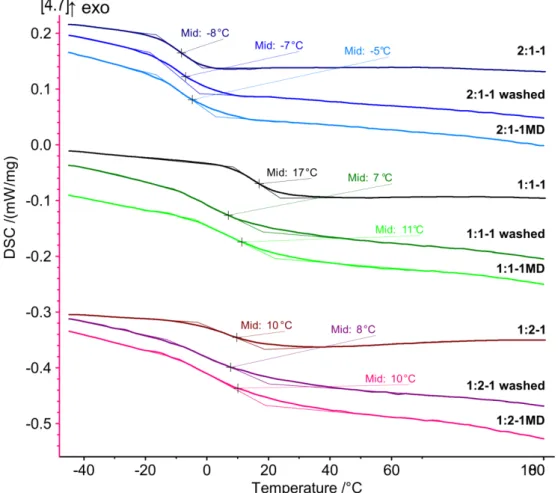
The difference in mechanical properties suggests a difference also in the thermal properties of the networks. To verify this hypothesis, the networks were analyzed by differential scanning calorimetry (DSC). For the softest network 2:1-1, a glass transition temperature (Tg) of -8 °C was found, whereas the Tg of the stiffer networks were closer to room temperature (Figure S8).
Interestingly, washing of the 1:1-1 networks resulted in a decrease in Tg by several degrees (Table 1). With the loss of a large amount of residual monomer and oligomers highly unlikely, we suggest a decreased ability to cope with the mechanical strain during swelling of this more rigid network with a higher degree of crosslinking density due to the stochiometric ratio of vinyl and thiol functionalities. Accordingly, no such difference was observed for networks 2:1-1 or 1:2-1.
Photochromic DASA Networks
The colorless and optically transparent precursor networks were developed into colorful DASA-networks by reaction with FAs. Colorful network structures were obtained as shown with the example of 2:1-1MD in Figure 2A. Monitoring this reaction with 2:1-15 using FTIR revealed that the reaction takes up to several days and no further reaction occurred after ~7 d (Figure S8). Due to the relatively slow reaction, which is typical for 2nd generation DASAs based on aromatic amines with low nucleophilicity,[22, 25] the degree of DASA
10
functionalization can be adjusted not only by the amount of EnePre in the precursor network but also by the duration for the DASA development step. In fact, we limited the reaction time for several samples since fully reacted DASA-networks from a 100:1 ene-EnePre ratio often were already too intense in color to be analyzed by UV-vis spectrometry. Variation of the FA type enabled the generation of three different DASA-networks with peak absorbance values of 562 nm (MD), 590 nm (BD), and 616 nm (PD), covering an overall absorbance range of more than 200 nm (Figure 2B). Importantly, photoswitching occurred for all network compositions and for all DASA types with the complete loss of color under white light irradiation and thermal reversion to the colored state in the dark (Figure S9/10). Figure 2C shows the loss of characteristic absorption peak of DASA in the 2:1-1MD network upon white light irradiation.
Figure 2. (A) Schematic depiction of the photoisomerization of a DASA-containing network (MD) with open (colorful) and closed (colorless) state. The photos on the right show images of network before and after development (2:1-1 and 2:1-1MD). (B) UV-vis spectra of three different DASA-networks derived from one precursor network. The networks are partially photoswitched to reduce the peak intensity to the range of the spectrometer.
(C) Spectra of 2:1-1MD before and after incremental photoswitching by white light irradiation (each spectrum corresponds to additional 10 s of irradiation). (D) Back-isomerization in the dark of photoswitched DASA-networks of dif-ferent composition. Due to the high absorbance values, the recovery could not be monitored from the peak wavelength for all networks (560 nm for 2:1-1MD, 586 nm for 1:1-1MD and 520 nm for 1:2-1MD). Yellow line indicates point of irradiation. (E) Cycling of 2:1-1MD between colorful and colorless state by repeated irradiation (30 s) and recovery in the dark (120 min).
11
In our previous work, we found a strong dependency of both the DASA photoswitching and the thermal back-isomerization on the Tg of the matrix polymer, with a rigid matrix impeding or even preventing it entirely.[25] As described above, all networks possessed glass transitions below room temperature, and the Tg for the 2:1-1 network was increased from -8 °C to -5 °C after the FA reaction (Table 1, Figure S7). To investigate the influence of matrix mechanics on the DASA behavior, we monitored the thermal recovery in the dark for the different network compositions. Interestingly, the recovery rate was found to strongly depend on the specific network type (Figure 2D and Table 1): the t3/4 (the recovery time of 75 % of the initial absorbance) increased from the soft matrix to 1:1-1MD as the most rigid and crosslinked network, suggesting that the soft network favors a fast response. In addition to the network composition, the structure of DASAs also influenced the recovery rate: the Pyra-based PD recovers faster in comparison to BD and to MD (Figure S10D). These results are in line with our previous findings[22, 25] and underline the importance of tuning the matrix properties as well as carefully choose the DASA type to achieve the desired photoswitching performance.
DASAs are generally susceptible to degradation by strong nucleophiles and, accordingly, free thiols in the networks can result in the degradation of part of the DASA population during the development step or even over time.[12] Indeed, we found an increase in color intensity from the 2:1-1MD network over 1:1-1MD to network 1:2-1MD (Figure S9). The last network has the highest color intensity presumably due to the absence of thiols that may induce DASA degradation. Accordingly we could in the future produce soft networks without residual thiols from a super-stoichiometric amount of thiol. One possibility would be co-macromonomers, for example ene-functional silicones, that increase the softness of the network due to a plasticizing effect.
Nanoscribe 3D-Printing and Dynamic Tuning
With the superior properties of the 2:1-1 network for photoswitching experiments, we chose this ink for nanoscale 3D-printing of photochromic objects via 2PP. To this end, a commercial
12
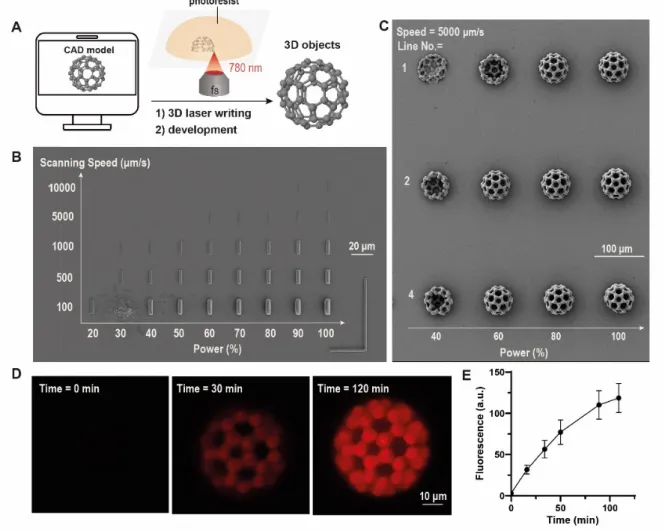
Nanoscribe GT 3D microprinter was applied to screen the processing window of this ink and to fabricate photochromic objects with user-dictated complex structures (Figure 3A). As shown in Figure 3B, we firstly fabricated simplified line-like structures at varying scanning speeds (100 µm/s – 10 mm/s) and laser power (7.2 mW to 36 mW). The robust thiol-ene reactions enabled rapid controlled fabrication of reproducible microstructures at writing speeds up to 50 mm/s, which is the upper limit of the device.
Figure 3. Nanoscribe 3D-printing, functionalization and photo-switching of color-changing fullerene-shaped microstructures. A) Schematic of direct laser writing using a C60-shaped CAD model; B-C) SEM images of micro- printed rectangular structures at varying scanning speed and laser power (B) and complex C60 microstructures at varying line number and laser power (C); D-E) After development, functionalization and laser excitation, the C60 microstructures are photoswitchable. D) Microscopic images of the microstructures at different time points after laser excitation. E) The thermal recovery kinetics of DASA-containing networks could be precisely monitored by the fluorescence.
Furthermore, we attempted to fabricate more complex 3D micro-objects using a C60-shaped CAD model. Since this model has a spherical shape, the initially printed microstructures tend to collapse or distort, presumably due to the limited interfacial bonding depth (< 1 µm) despite
13
the use of thiol-functionalized glass slide. To address this, we optimized the printing process by increasing the bonding depth to 5 µm to enhance the stability of microprinted structures.
Furthermore, we found that the increase of LineNumber from 1 to 2 and to 4 could also greatly enhance the structural stability after development in acetone (Figure 3C).
Finally, we studied the feasibility of incorporating DASA into the 3D microprinted networks as well as the photoswitching properties thereof. The printed microstructures were reacted with one type of FA (Meld) for 12 h under dark to incorporate the DASA into the network. The shortened duration for this reaction is based on the fact the structures are porous structures with sizes of ca. 50 µm, thereby facilitating the FA reaction. Unlike the samples prepared by 1PP, the printed microstructures have a different appearance. This is likely due to the variations in photoinitiation kinetics and photon intensity between 1PP and 2PP.
Due to the small scale of these structures that renders UV-Vis spectrometry unavailable for their investigation, we decided to employ the fluorescence of DASAs to monitor photoswitching and recovery of nano-3D-printed photochromic structures. Mason et al. have previously shown that the fluorescence of the open form of alkylamine-based DASAs can be used to track the closed-open transition, though this work did not include reversible photoswitching.[21] To our knowledge, no previous work has employed the change in DASA fluorescence to monitor their photoswitching transition in solution or covalently immobilized in a polymeric matrix. Yet it presents a method towards quantitative information on the DASA state even in miniscule intricately and complex structures. First, we investigated the fluorescence of the aromatic amine-based DASA in solution and in 2:1-1MD networks. We found that the DASA solutions and networks have weak fluorescence in the open state (Figure S12), with max. fluorescence of DASA in the open state at 615 nm. Once irradiated with white light, the fluorescent intensity of DASA disappeared due to the transition into the colorless and non-fluorescent closed state upon switching. Furthermore, the recovery of fluorescence in
14
DASA networks reached >95% of the fluorescence before switching, and 100% in DASA solutions (Figure S12), much in accordance with our previous UV-Vis experiments.
We applied these findings to characterize the switching behavior of our structures. The 3D microprinted fullerene structures were, first, photobleached by green laser irradiation at 546 nm for 2 min on a Zeiss LSM880 laser microscope. Subsequently, time-lapse confocal imaging was performed to monitor the fluorescence recovery of DASA-containing networks inside the microstructure. As shown in Fig. 3D, the fluorescence of DASA in the microstructure recovers after 120 min. Moreover, the fluorescence intensity could be quantified and plotted as depicted in Fig. 3E. To the best of our knowledge, this is the first work that demonstrates the fluorescence of DASA photoswitches can be utilized to study the reversible switching and recovery of these photochromes, allowing the reading of DASA-containing polymer matrices in 3D.
Conclusions
Visible light-responsive polymer networks with covalently attached DASA photoswitches were accessed in two steps via photocrosslinking of a thiol-ene clickable ink into networks and subsequent development of a range of DASAs with different color profiles. Photocrosslinking with UV photoinitiation provided thin films while 2PP with near infrared laser pulses enabled the microfabrication of 3D objects. The properties of the networks as a photoswitch matrix depended strongly on the stoichiometry of the monomers and the resulting mechanical properties of the polymer networks, with softer networks enabling faster DASA isomerization.
The presented methodology provides control over the composition and the geometry of the networks, as well as the degree of functionalization and type of integrated DASAs. Integration of new monomers and comonomers will allow further improvement of the matrix properties in the future to provide optimized photoswitching properties, e.g., by using ene-functionalized silicones. Importantly, the modular nature of the presented approach can be readily expanded with new FAs, or combinations thereof, to design absorbance and photoswitching profiles over the whole visible spectrum. In the future, the combination with fluorescent dyes in the matrix
15
can allow both 2-photon-based writing and fluorescence-based reading. With the optimization of the 2PP process on high efficiency commercial microprinters, the presented methodology can enable the writing of photochromic objects for applications as smart multi-addressable optical filters and switches within complex microfabricated structures, with high demands on their exact localization and shape.
Supporting Information
Supporting Information is available from the Online Library or from the author.
Acknowledgements
The Swiss National Science Foundation (SNSF) is acknowledged for the financial support through Grant No. 200021_172609. XHQ would like to acknowledge ETH Zurich and Swiss National Science Foundation (SNSF) for the financial support through an ETH Career Seed grant (SEED21 18-2) and a SNSF Spark grant.
References
[1] Crano, J. C. & Guglielmetti, R. J. Organic Photochromic and Thermochromic Compounds, vol.
1: Main Photochromic Families of Topics in Applied Chemistry (Kluwer Academic Publishers, New York, 1999), 1 edn.
[2] Russew, M. M. & Hecht, S. Photoswitches: From molecules to materials. Adv. Mater. 22, 3348–
3360 (2010).
[3] Tian, H. & Zhang, J. (eds.). Industrial Applications and Perspectives (Wiley-VCH, Weinheim, 2016).
[4] Pauly, A. C. et al. ATRP-based synthesis and characterization of light-responsive coatings for transdermal delivery systems. Sci. Technol. Adv. Mater. 16, 034604 (2015).
[5] Schöller, K. et al. From Membrane to Skin: Aqueous Permeation Control Through Light Responsive Amphiphilic Polymer Co-Networks. Adv. Funct. Mater. 24, 5194–5201 (2014).
[6] Irie, M. Diarylethenes for memories and switches. Chem. Rev. 100, 1685–1716 (2000).
[7] Wang, L. & Li, Q. Photochromism into nanosystems: towards lighting up the future nanoworld.
Chem. Soc. Rev. 47, 1044–1097 (2018).
[8] Xiao, P., Zhang, J., Zhao, J. & Stenzel, M. H. Light-induced release of molecules from polymers.
Prog. Polym. Sci. 74, 1–33 (2017).
[9] Bleger, D. & Hecht, S. Visible-light-activated molecular switches. Angew. Chem. Int. Ed. Engl.
54, 11338–11349 (2015).
[10] Helmy, S. et al. Photoswitching using visible light: A new class of organic photochromic molecules. J. Am. Chem. Soc. 136, 8169–8172 (2014).
16
[11] Helmy, S., Oh, S., Leibfarth, F. A., Hawker, C. J. & Read de Alaniz, J. Design and synthesis of donor-acceptor stenhouse adducts: A visible light photoswitch derived from furfural. J. Org.
Chem. 79, 11316–11329 (2014).
[12] Lerch, M. M., Szymanski, W. & Feringa, B. L. The (photo)chemistry of stenhouse photoswitches:
guiding principles and system design. Chem. Soc. Rev. 47, 1910–1937 (2018).
[13] Yan, Q. et al. Visible light responsive donor-acceptor stenhouse adducts with indoline-tri/tetra- phenylethylene chromophore: Synthesis, aggregation-induced emission, photochromism and solvent dependence effect. Dyes Pigm. 178, 108352 (2020).
[14] Hemmer, J. R. et al. Tunable visible and near infrared photoswitches. J. Am. Chem. Soc. 138, 13960–13966 (2016).
[15] Hemmer, J. R. et al. Controlling dark equilibria and enhancing donor-acceptor stenhouse adduct photoswitching properties through carbon acid design. J. Am. Chem. Soc. 140, 10425–10429 (2018).
[16] Mostafavi, S. H. et al. Photoinduced deadhesion of a polymer film using a photochromic donor- acceptor stenhouse adduct. Macromolecules 52, 6311–6317 (2019).
[17] Yap, J. E., Mallo, N., Thomas, D. S., Beves, J. E. & Stenzel, M. H. Comparing photoswitching of acrylate or methacrylate polymers conjugated with donor-acceptor stenhouse adducts. Polym.
Chem. 10, 6515–6522 (2019).
[18] Ulrich, S. et al. Electrospun colourimetric sensors for detecting volatile amines. Sens. Actuators, B 322, 128570 (2020).
[19] Chen, Y. et al. Visible light-controlled inversion of pickering emulsions stabilized by functional silica microspheres. Langmuir 34, 2784–2790 (2018).
[20] Jia, S. et al. Investigation of donor-acceptor stenhouse adducts as new visible wavelength-
responsive switching elements for lipid-based liquid crystalline systems. Langmuir 33, 2215-2221 (2017).
[21] Mason, B. et al. A temperature-mapping molecular sensor for polyurethane-based elastomers.
Appl. Phys. Lett. 108, 041906 (2016).
[22] Rifaie-Graham, O. et al. Wavelength-selective light-responsive DASA-functionalized polymersome nanoreactors. J. Am. Chem. Soc. 140, 8027–8036 (2018).
[23] Senthilkumar, T. et al. Conjugated polymer nanoparticles with appended photo-responsive units for controlled drug delivery, release, and imaging. Angew. Chem. Int. Ed. Engl. 57, 13114–13119 (2018).
[24] Sinawang, G., Wu, B., Wang, J., Li, S. & He, Y. Polystyrene based visible light responsive polymer with donor-acceptor stenhouse adduct pendants. Macromol. Chem. Phys. 217, 2409–
2414 (2016).
[25] Ulrich, S. et al. Visible light-responsive dasa-polymer conjugates. ACS Macro Lett. 6, 738–742 (2017).
[26] Wu, B. et al. Visible light triggered aggregation-induced emission switching with a donor- acceptor stenhouse adduct. J. Mater. Chem. C 6, 8538–8545 (2018).
[27] Chen, S., Li, W. & Zhu, W. Multi-addressable Photochromic Materials, 71–108 (Wiley-VCH, Weinheim, 2016).
[28] Lerch, M. M., Hansen, M. J., Velema, W. A., Szymanski, W. & Feringa, B. L. Orthogonal photoswitching in a multifunctional molecular system. Nat. Commun. 7, 12054 (2016).
[29] Murphy, S. & Atala, A. 3d bioprinting of tissues and organs. Nat. Biotechnol. 32, 773–785 (2014).
[30] Qin, X.-H., Ovsianikov, A., Stampfl, J. & Liska, R. Additive manufacturing of photosensitive hydrogels for tissue engineering applications. BioNanoMaterials 15, 49–70 (2014).
[31] Wallin, T., Pikul, J. & Shepherd, R. 3d printing of soft robotic systems. Nature Reviews Materials 3, 84–100 (2018).
17
[32] LaFratta, C., Fourkas, J., Baldacchini, T. & Farrer, R. Multiphoton fabrication. Angew. Chem. Int.
Ed. Engl. 46, 6238–6258 (2007).
[33] Torgersen, J. et al. Hydrogels for two-photon polymerization: A toolbox for mimicking the extracellular matrix. Adv. Funct. Mater. 23, 4542–4554 (2013).
[34] Qin, X.-H., Wang, X., Rottmar, M., Nelson, B. J. & Maniura-Weber, K. Near-infrared light- sensitive polyvinyl alcohol hydrogel photoresist for spatiotemporal control of cell-instructive 3d microenvironments. Adv. Mater. 30, 1705564 (2018).
[35] Hoyle, C. E., Lowe, A. B. & Bowman, C. N. Thiol-click chemistry: a multifaceted toolbox for small molecule and polymer synthesis. Chem. Soc. Rev. 39, 1355–1387 (2010).
[36] Iha, R. K. et al. Applications of orthogonal "click" chemistries in the synthesis of functional soft materials. Chem. Rev. 109, 5620–5686 (2009).
[37] Kumpfmueller, J. et al. Two-photon-induced thiol-ene polymerization as a fabrication tool for flexible optical waveguides. Des. Monomers Polym. 17, 390–400 (2014).
[38] Qin, X., Wu, Y., Wang, K., Tan, H. & Nie, J. In-situ synthesis of exfoliated nanocomposites by photopolymerization using a novel montmorillonite-anchored initiator. Appl. Clay Sci. 45, 133–
138 (2009).
[39] Morimoto, M. et al. Photochromic Bulk Materials (2016).
[40] Shima, K., Mutoh, K., Kobayashi, Y. & Abe, J. Relationship between activation volume and polymer matrix effects on photochromic performance: Bridging molecular parameter to macroscale effect. J. Phys. Chem. A 119, 1087–1093 (2015).
[41] Evans, R. A. et al. The generic enhancement of photochromic dye switching speeds in a rigid polymer matrix. Nat. Mater. 4, 249–253 (2005).
[42] Boesel, L. F., Reis, R. L. & San Román, J. Innovative approach for producing injectable, biodegradable materials using chitooligosaccharides and green chemistry. Biomacromolecules 10, 465–470 (2009).
18 Table 1. Properties of precursor and DASA-networks.
Network* Thiol:
Enea
EneMonomer: EnePrea
Tg [°C] b
SVol (acetone)
G’/G’’ c [MPa]
tanδ c G* c [MPa]
t3/4d (min)
2:1-0 £ 2:1 100:0
Data was not acquired for these formulations 2:1-15 £ 2:1 100:15
2:1-3 £ 2:1 100:3
2:1-1 2:1 100:1 -8/-7/-5 1.5±0.03 1.5±0.2/
0.15±0.01 0.10±0.01 1.5±0.2 74 1:1-1 1:1 100:1 17/7/11 1.2±0.02 2.9±0.9/
1.8±0.4 0.61±0.13 3.4±0.1 111 1:2-1 1:2 100:1 10/8/10 1.4±0.02 2.9±0.2/
1.5±0.01 0.51±0.03 3.2±0.2 159
aRatio in monomer mixture. b Determined by DSC before / after washing of network / after DASA development with Meld. c Determined by in situ photorheology and dynamic rheology.d Time required to reach 75 % of the final absorbance value during the recovery experiments (Figure 2D).
* The networks are named based on their composition. For example, 2:1-1 refers to a network formed by a monomer mixture with a thiol-to-ene ratio of 2:1, and a 0.01 molar equivalent of EnePre to the ene functional groups of the monomer.
£ These networks were used to investigate the influence of particular aspects and are described in the supporting information. E.g., a network with such a high DASA concentration as in the network 2:1-15 had little practical relevance of its own, but was synthesized to increase the visibility of key peaks for the FTIR analysis.
19
Supporting Information
Nano-3D-printed Photochromic Objects
Sebastian Ulrich1,3, Xiaopu Wang2,6, Markus Rottmar4, René Michel Rossi1,Bradley J. Nelson2, Nico Bruns3, Ralph Müller5, Katharina Maniura-Weber4, Xiao-Hua Qin4,5,* and Luciano Fernandes Boesel1
1 Empa, Swiss Federal Laboratories for Materials Science and Technology, Laboratory for Biomimetic Membranes and Textiles
Lerchenfeldstrasse 5, 9014 St. Gallen, Switzerland
2 Institute of Robotics and Intelligent Systems ETH Zurich
Tannenstrasse 3, 8092 Zurich, Switzerland
3 Adolphe Merkle Institute University of Fribourg
Chemin des Verdiers 4, 1700 Fribourg, Switzerland
4 Empa, Swiss Federal Laboratories for Materials Science and Technology Biointerfaces
Lerchenfeldstrasse 5, 9014 St. Gallen, Switzerland
5 Institute for Biomechanics ETH Zurich
Leopold-Ruzicka-Weg 4, 8093 Zurich, Switzerland
6 Shenzhen Institute of Artificial Intelligence and Robotics for Society The Chinese University of Hong Kong, Shenzhen
518172 Shenzhen, China E-mail: qinx@ethz.ch
20
Experimental section
Materials
All solvents including anhydrous solvents were purchased from Sigma-Aldrich or Fisher Scientific and were of analytical or reagent grade. All chemicals were purchased from Sigma Aldrich or TCI Europe unless stated otherwise and were of analytical or reagent grade. 1,3,5-Triallyl-1,3,5-triazine- 2,4,6(1H,3H,5H)-trione (3Ene) and pentaerythritol tetrakis(3-mercaptopropionate) (4Thiol) were used as the multifunctional ene and thiol monomer, respectively. Diphenyl(2,4,6- trimethylbenzoyl)phosphine oxide (TPO) was used as the photoinitiator for both single-photon and multiphoton polymerization.
Synthesis of Ene-Functional Aromatic Amine DASA Precursor (EnePre)
Scheme S1. Synthesis of EnePre.
In a round-bottom flask equipped with a magnetic stir bar, para-anisidine (2.60 g, 21.1 mmol, 1 eq) was dissolved in 80 mL of anhydrous dichloromethane under nitrogen atmosphere, followed by the addition of triethylamine (4.40 mL, 31.5 mmol, 1.5 eq). Under ice cooling, 4-pentenoyl chloride (2.33 mL, 21.1 mmol, 1 eq) dissolved in 10 mL of anhydrous dichloromethane was added slowly to the solution over 30 min, followed by stirring at room temperature for 30 min, after which the reaction was deemed complete (TLC, heptane/ethylacetate 3:2). The reaction mixture was diluted with 50 mL of dichloromethane and washed with 1 M HCl (2x, 50/30 mL), 1 M NaOH (30 mL), and saturated NaCl (30 mL) solutions. The organic phase was passed through 3 cm of silica, dried over anhydrous Na2SO4 and the solvent was removed in vacuo to yield 3.40 g (79 %) of N-(4-methoxy-phenyl)-pent-4-enamide as a brown-yellow solid. It was directly used without further purification and characterization for the next step.
N-(4-methoxy-phenyl)-pent-4-enamide (3.00 g, 14.6 mmol, 1 eq) was dissolved in 20 mL of anhydrous tetrahydrofuran under nitrogen atmosphere in a round-bottom flask equipped with a magnetic stirrbar. A 1 M solution of lithium aluminum hydride in THF (30 mL, 30 mmol, 2.1 eq.) was added dropwise under ice cooling. Subsequently, the reaction was allowed to warm up to room temperature and stirred overnight. The next day, additional of lithium aluminum hydride solution (10 mL, 10 mmol) was added and the reaction was stirred at 40 °C for 2 h, after which the reaction was deemed complete by TLC (dichloromethane/methanol 19:1). The reaction was quenched by slow addition of 30 mL of water under ice cooling. Subsequently, 50 mL of diethylether were added, followed by 10 mL of 1 M NaOH. After a sediment had formed, the organic phase was decanted, filtered and dried over anhydrous Na2SO4, followed by evaporation of the solvent in vacuo to yield 2.32 (83 %) of the vinyl-functional aromatic amine precursor (EnePre) 4-methoxy-N-(pent-4-en-1-yl)aniline as a brown-black oil. 1H NMR (400 MHz, CDCl3) δ/ppm: 6.80-6.77 (m, 2H), 6.60-6.6.56 (m, 2H), 5.89-5.79 (m,
O
NH2
O
HN Cl
+ O TEA
DCM
LiAlH4
THF O
HN O
21
1H), 5.08-4.98 (m, 2H), 3.75 (s, 3H), 3.09 (t, J = 7.1 Hz, 2H), 2.17 (q, J = 7.1 Hz, 2H), 1.71 (pentet, J = 7.3 Hz, 2H) (The NMR spectrum with peak assignment is presented in Figure S1). ATR-FTIR (wavenumber/cm-
1): 3386 (N-H), 1508 (CAr-N), 1230 (CAr-O) (FTIR spectrum in Figure S2)
Photoink Preparation
The photoink formulation consists of 4Thiol and 3Ene monomers mixed in a thiol-ene stoichiometric ratio of 1:2, 1:1, and 2:1, respectively. TPO photoinitiator was added into the formulation at 3 wt%. In a typical procedure, 3Ene was firstly transferred into a brown vial under light protection and then mixed with 100 µL of toluene containing TPO and EnePre. The resultant formulation was sonicated for 15 min, followed by addition with appropriate amounts of 4Thiol. The formulation was sonicated for another 15 min before use.
UV Photocrosslinking
Cylindrical polymer pellets were prepared by UV photopolymerization of the thiol-ene formulation.
Briefly, the formation was added to a custom-made multi-well Teflon mold (diameter, 6 mm; thickness, 0.7 mm) and covered with No. 1.5 cover slip. The samples were then transferred to a UV lamp (UVASPOT 400/T, 400 W, 315 nm long pass filter, Dr. Hönle AG, Germany) and irradiated for 3 min.
Afterwards, the pellets were separated from the mold and stored in a desiccator for further studies. The 100 µm thin films were prepared in the in situ photorheology setup and removed by soaking in acetone.
2PP Microfabrication
Microfabrication of 3D objects were performed by means of two-photon polymerization on a Zeiss LSM 780 microscope (10x objective, 780 nm) coupled with a femtosecond Mai Tai DeepSee laser. Twenty microliter of the photoresist was loaded into a PDMS well (diameter, 4 mm; thickness, 1 mm) on a glass dish (MatTek 35 mm, No. 1.5). The glass bottom was functionalized with sulfhydryl groups by surface treatment using 3-(mercaptopropyl)trimethoxysilane to facilitate covalent fixation of microfabricated objects to the glass. Through 3D laser scanning, localized polymerization was induced inside the formulation within region of interests (ROI). Cubical structures were obtained accordingly after washing the samples in ethanol for 10 min.
DASA Development of Pellets, Thin Films and 2PP Structures
MD Development with Meld: Typically, pellets or thin films were immersed in a solution of Meld in acetone (100 mg∙mL-1, 0.45 mmol∙mL-1) in a screw cap vial and left to react in the dark. Subsequently, the pellets and thin films were washed in acetone for 3 d and 1 d, respectively, and subsequently dried in vacuo. Structures from 2P polymerization were treated analogously, but the reaction was shortened to 2 d to yield a lower DASA concentration.
BD Development with Barb: The thin films were immersed in a solution of Barb in acetone (71 mg∙mL-1, 0.22 mmol∙mL-1) and left to react for either 10 d for 2:1-1Barb or 3 d for 1:2-1Barb to achieve a
22
lower DASA concentration. Subsequently, the thin films were washed in acetone for 1 d and dried in vacuo.
PD Development with Pyra: The thin films were immersed in a solution of Pyra in THF (35 mg∙mL-
1, 0.11 mmol∙mL-1) and left to react for 5 d.
ATR-FTIR Analysis
Attenuated total reflection Fourier-transform infrared (ATR-FTIR) spectra were recorded on a Varian 640-IR FT-IR spectrometer equipped with a diamond crystal insert. Spectra were recorded with a resolution of 8 cm-1. Typically, if not otherwise stated, washed and dried pellets were analyzed.
In situ FTIR Monitoring
A drop of the precursor solution was placed on the ATR crystal and a glass cover slip was placed on top. An optical fibre UV light source (Omnicure S1000, 100W, Excelitas Technologies Corp., Canada) was placed 7 cm above the crystal. Spectra were continuously recorded with a resolution of 16 cm-1 over 110 min with a time resolution of 1.4 s. The polymerization was initiated by switching on the UV light source after the recording of the 13th spectrum was completed (18.2 min) and the light source was turned off after the recording of the 56th spectrum was finished (78.3 min). To monitor the kinetics of the reaction, the intensity at the wavelengths 3075 cm-1 (vinyl C-H, decrease), 2927 cm-1 (alkyl C-H, increase), 2562 cm-1 (thiol S-H, decrease), and 924 cm-1 (vinyl, decrease) were monitored relative to a baseline that was calculated for every measured spectrum from a straight line between the values at 3200 cm-1 and 2700 cm-1 (no peaks).
NMR Spectroscopy
The 1H NMR spectra were recorded at 400.2 on a Bruker Avance III 400 NMR spectrometerv at 297.2 K using the standard Bruker pulse programs and parameter sets. The 1H NMR spectra were referenced internally with residual resonances of the solvent CDCl3.
In situ Photorheology
The photo-reactivity and mechanical properties of thiol-ene formulations were evaluated on a photo- rheometer device (Anton-Paar, MCR301, Austria) as described elsewhere.1 The light source was the same as for the in situ FTIR experiment. Specifically, time-resolved rheometric analysis was performed at a constant angular frequency of 10 rad/s and a constant strain of 0.5 %. Three parameters are determined from a rheological analysis: elastic modulus (G’), loss modulus (G”), and loss factor (tan δ)
= G”/G’. Dynamic strain-sweep measurements were performed to determine the linear viscoelastic (LVE) range at an fixed angular frequency of 10 rad/s and strain of 0.01-200 %. For dynamic frequency- sweep, the samples were analyzed at constant strain of 0.5% and angular frequency of 1-300 rad/s.
23 DSC Analysis
DSC curves were recorded on a NETZSCH DSC 214 Polyma (NETZSCHZ, Germany) under nitrogen atmosphere at a heating rate of 10 K min-1 and a cooling rate of 20 K min-1. It was analyzed using the associated software (NETZSCH Proteus Thermal Analysis, Version 7.1.0). A first heating cycle was recorded for the pellets from -65 °C to 120 °C, followed by a second heating cycle from -65 °C to160 °C.
Glass transition temperatures were determined for the second heating cycle as the mid-point of the observed transitions.
UV-vis Measurements and Photoswitching of Thin Films
Measurement of UV-vis spectra and monitoring of the recovery after photoswitching were recorded on a Cary 50 Bio UV-visible spectrophotometer. Thin films were placed on the transparent plastic lid of a well plate for analysis. For photoswitching experiments, the thin films on the well plate were placed directly above the LED of a Motorola Moto X Play (Motorola, USA, intensity 1519 mW∙m-2, spectrum shown in Figure S3) and irradiated, typically for 30 s. If the peak intensity of a film exceeded the device’s limitation, the film was partially photoswitched before recording the spectra to reduce the peak absorbance into the range of the device.
Swelling Analysis
For swelling experiments, round pellets (700 µm thickness) were placed in the solvent for several days. The images of the pellets were taken in the dry and in the swollen state by way of an optical microscope. The diameter of the pellets was measured independently at four different points using ImageJ software (Version 1.51n) and the volumetric degree of Swelling SVol was calculated as:
𝑆𝑆Vol = 14∑ �∅swollen∅
dry �3
4𝑖𝑖=1 Equation S1
Light microscopy
Light images were recorded on a Keyence VHX-1000 system (Keyence Corporation of America, USA) from a 5° angle for 2PP objects and 0° for swelling analysis.
Microscale Color-Tuning and Photopatterning
Microscale color-tuning of Dasa-functionalized polymer networks was realized by single-photon laser scanning at 560 nm on a LSM 780 microscope as aforementioned. Briefly, 60 µm z-stacks of circle, square or triangle was sequentially created inside the two-photon microfabricated objects within ROI.
After each photopatterning experiment, a microscopic image was captured by using a Axio phase- contrast transmission camera. To determine recovery kinetics, the intensity was monitored via time- lapse experiment with time interval of 30 s for 30 min.
24 Multiphoton Lithography
For 3D microfabrication of complex objects, the photoresist was structured on a Photonic Professional GT micro-printer (Karlsruhe, Germany). Briefly, a glass slide was secured to the sample holder with glue, on the backside of the glass slide a drop of oil was dropped at the center, and on the frontside of the glass slide a drop of the photoresist was dropped at the center. The sample was then transferred into the micro-printer for 3D printing. Several 3D structures (3D octopus, church, and fullerene-like lockyball structures) were designed and fabricated using the photoresist. The fabrication code was generated by processing the 3D structure models with the software ‘Describe’. The fabrication parameters, laser power and scanning speed are 72 mw and 50 mm/s, respectively. The wavelength of the laser pulse used in this micro-printer is 780nm, and the objective used is 63×(NA1.4). After the photopolymerization, the sample was developed in acetone for several minutes to remove the un- polymerized photoresist and wash the printed 3D structures.
During the fabrication, the interface between the photoresist and the glass slide was hard to find.
This indicates that the refractive index difference between the photoresist and the glass slide is not sufficient (≤0.05). To make sure the fabricated structures are attached to the glass slide, the interface between the glass slide and air nearby the photoresist droplet was found and the interface between the photoresist and the glass slide was assumed to be at the same height.
25
Supplementary Figures
1H NMR Spectrum of EnePre
Figure S1. 1H NMR spectrum of EnePre. Residual solvent signals (CDCl3 and water) are indicated by an asterisk).
26 FTIR Spectra of Network Components
Figure S2. FTIR spectra of network components compared to a 2:1-15 network: 3Ene monomer, 4Thiol monomer, and aromatic amine DASA precursor EnePre. Key signals of the individual components that appear in the network spectra are marked with black lines.
27 Emission Spectrum of White Light Source
Figure S3. Emission profile of the white light source, the LEDs of a Motorola MotoX Play mobile phone.
Washing of Precursor Networks
Figure S4. FTIR spectra of networks as synthesized and after washing in ethylacetate.
28 Variation of EnePre Incorporation
Figure S5. FTIR spectra of networks of equal monomer ratio but different amounts of EnePre. A characteristic peak of EnePre is marked with a black line.
29
In situ FTIR Monitoring of Photocrosslinking Kinetics
Figure S6. In situ FTIR monitoring of the network synthesis. (A) Spectra of different networks taken from the continuous FTIR monitoring at time points before UV initiation (10 min) and after the polymerization was complete (100 min). Key wavenumber values at which the polymerization can be monitored are marked with arrows. (B) In situ FTIR monitoring of network 2:1-1 at different wavenumbers. (C) In situ FTIR monitoring of different network with results from three independent experiments for each network.
30 In situ Photorheology
Figure S7.In situ photorheology of the resins. (A) Schematic of the photo-rheology. (B) The time- resolved plots showing the elastic modulus (G’) and loss modulus (G’’) of various samples with different thiol-ene ratio:UV irradiation started after 60 s, the measurement was at 1% stain and 10 rad/s. (C) The frequency sweep of the in situ cured samples with varying thiol-ene ratio at 1% stain, frequency 0.1-100 rad/s.
31 Differential Scanning Calorimetry
Figure S8. DSC analysis of networks before and after washing and after DASA development with Meld.
FTIR Monitoring of DASA Development
Figure S9. FTIOR monitoring of the DASA formation in network 2:1-15 with activated furan adduct Meld towards DASA network 2:1-15MD.
32 Photoswitching in Different Networks
Figure S10. Photographic images of DASA networks 2:1-1MD, 1:1-1MD, 1:2-1MD before and after photoswitching.
Different DASAs
Figure S11. (A/B) UV-vis spectra of DASA networks (A) 1:2-1BD and (B) 1:2-1PD before and after photoswitching. The residual absorbance does not indicate incomplete photoswitching but is due to the fast recovery in between irradiation and recording of the spectra. (C) Photographic images of the same networks before and after photoswitching. (D) Monitoring of the back-isomerization images of DASA networks 1:2-1MD (520 nm), 1:2-1BD (620 nm), 1:2-1PD (645 nm). Networks were completely photoswitched with a white light source and the thermal recovery in the dark was monitored by UV- vis spectroscopy.
33 DASA fluorescence
Figure S12. (A) Fluorescence intensity spectra of MD in solution before and after complete photoswitching and after complete recovery in the dark. (B) Fluorescence recovery in the dark of MD in solution, after photoswitching. (C) Fluorescence intensity spectra of films of 2:1-1MD before and after photoswitching and after recovery in the dark.
600 650 700 750 800 0
100 200
300 cycle2 before
cycle2 switched cycle2 recovered
Slit 10nm/10nm
Fluorescence Intensity [a.u.]
Wavelength λEx530nm [nm]
Initial state Photoswitched Recovered
0 50
0 100 200 300
SUP6A-DCM
Slit 10nm/10nm
Fluorescence @502nm (λEx530nm)
Time [min]
600 650 700 750 800 0
100 200 300 400 500 600 700
cycle2 before cycle2 switched cycle2 recovered
Slit 10nm/10nm
Fluorescence Intensity [a.u.]
Wavelength λEx530nm [nm]
Initial state Photoswitched Recovered
34 Nanoscribe: 2PP Microprinted DASA Octopus
Figure S13. Light microscopy image of a 2:1-1 octopus before and after DASA development with Meld into 2:1-1MD.
Dynamic tuning
Figure S14. (A) Schematic 3D microscale photoswitching. (B) 2:1-1MD cubicle (500x500 µm2, height 100 µm) before and during multi-step confocal laser photopatterning. The time in-between photopatterning steps allowed the back-isomerization in the previous photopatterns. (C) Monitoring of the back-isomerization and color recovery in a photoswitched ROI via the light transmission.