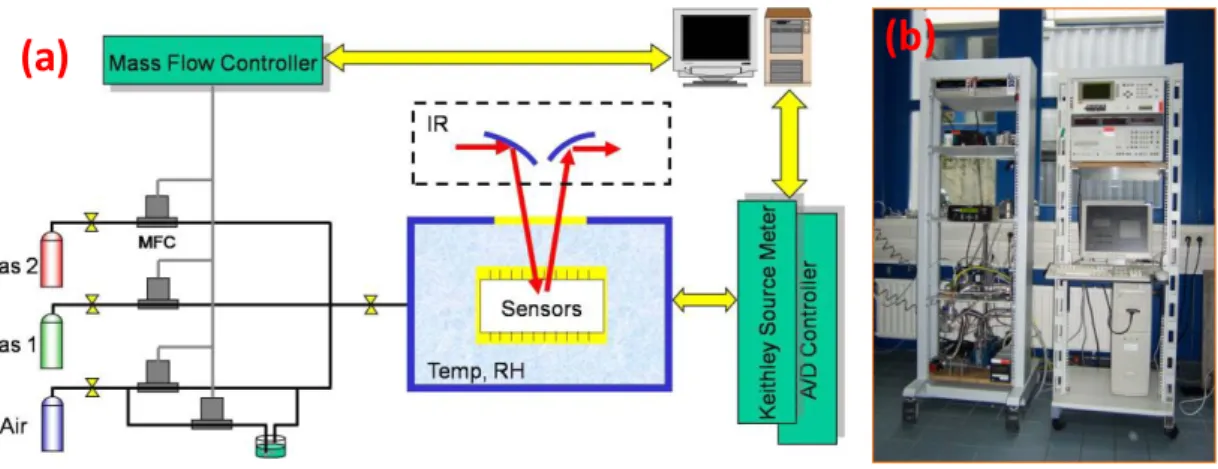
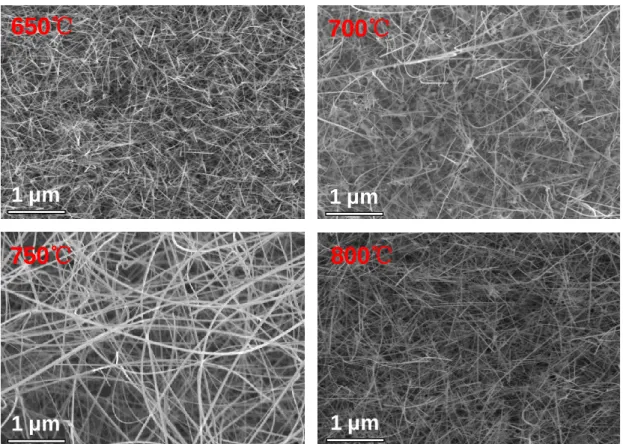
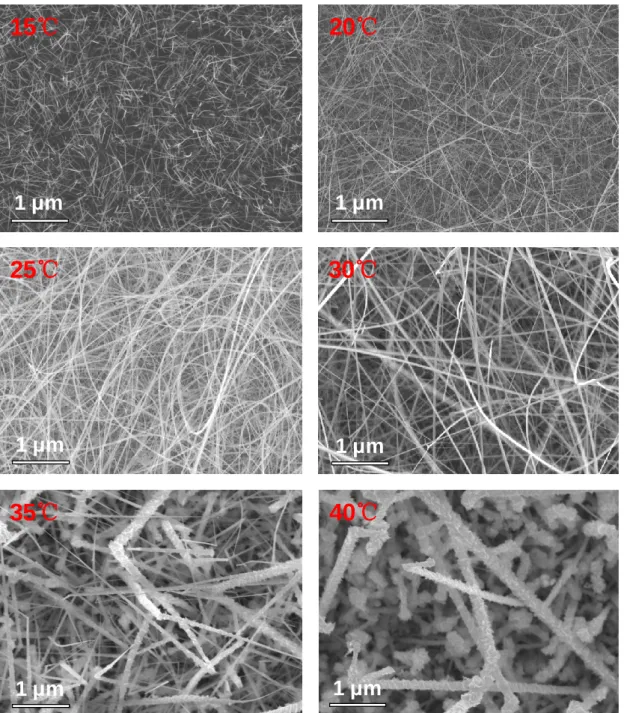
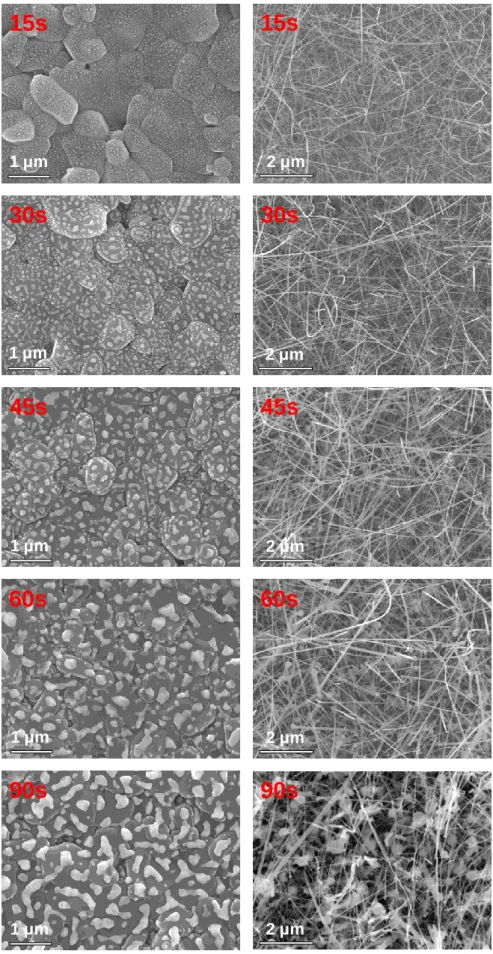
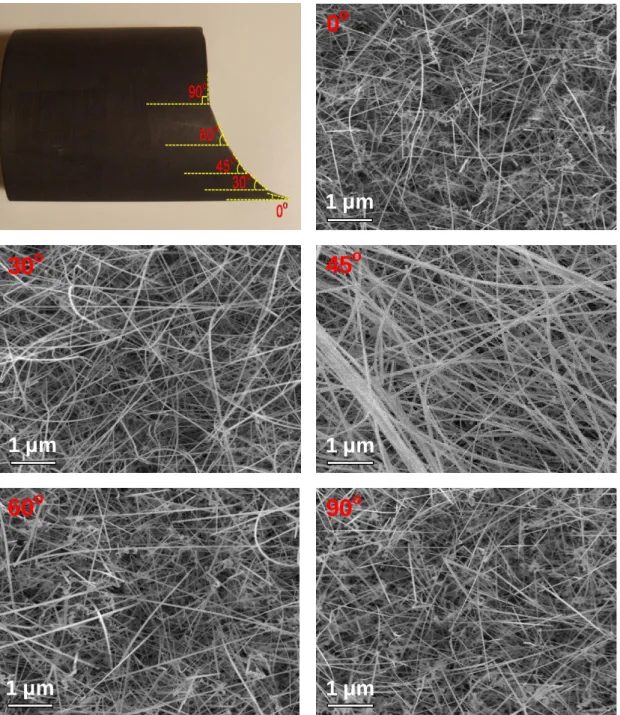
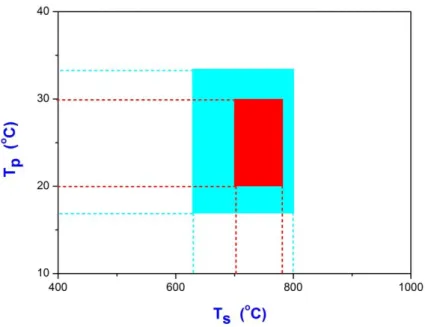
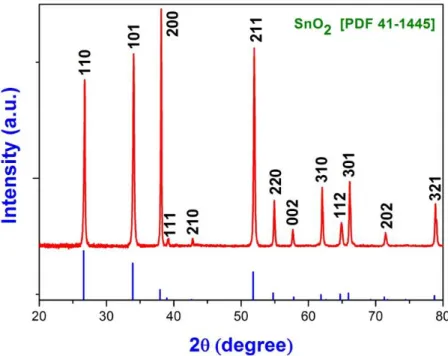
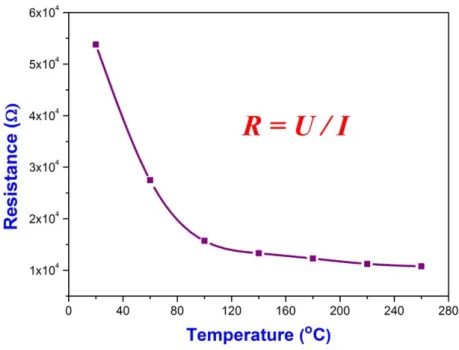
Chemical Vapor Deposition of
One Dimensional Tin Oxide Nanostructures:
Structural Studies, Surface Modifications and Device Applications
DISSERTATION
zur Erlangung des Doktorgrades
der Mathematisch-Naturwissenschaftlichen Fakultät der Universität zu Köln
vorgelegt von
Jun Pan
Köln, 2010
FIB-Tomographic Image of Ordered SnO2 Nanowires
Chemical Vapor Deposition of
One Dimensional Tin Oxide Nanostructures:
Structural Studies, Surface Modifications and Device Applications
DISSERTATION
zur Erlangung des Doktorgrades
der Mathematisch-Naturwissenschaftlichen Fakultät der Universität zu Köln
vorgelegt von
Jun Pan
Köln, 2010
FIB-Tomographic Image of Ordered SnO2 Nanowires