Ζ. Kristallogr. NCS 216 (2001) 5 0 3 - 5 0 4
© by Oldenbourg Wissenschaftsverlag, München
503
Crystal structure of barium dimagnesium dilithium disilicide, BaMg2Li2Si2, and of barium dimagnesium dilithium digermanide, Bal^M&Gez
F. Zürcher, S. Wengert and R. Nesper*
ΕΤΗ Zürich. Laboratorium für Anorganische Chemie, Universitätstraße 6, CH-8092 Zürich, Switzerland Received August 26. 2001, CSD-No. 409575 and CSD-No. 409576
jß J> Jß Jß
Mg
m m
j ^ B aφ
J J Jß Jß Jß Jß
Abstract
BaLi2Mg2Si2, trigonal, R3m (No. 166), a = 4.578(2) Ä, c = 26.05( 1) Ä, V = 472.9 Ä3, Ζ = 3, Rgl(F) = 0.009, wRK((F2) = 0.023, T= 293 K.
BaGe2Li2Mg2, trigonal, R3m (No. 166), a = 4.607(2) Ä, c = 26.14( 1) Ä, V = 480.5 Ä3, Ζ = 3, R&(F) = 0.019, wRobsfF2) = 0.054, T = 298 K.
Source of material
BaMg2Li2Si2 was prepared by heating the stoichiometric mixture of the elements at 1578 Κ (1 h, cooling down within 1 d) and forms brittle, dark silver, bar-like crystals. It is sensitive to air and moisture. BaMg2Li2Ge2 was prepared by heating the stoichio- metric mixture of the elements at 1273 Κ (1 d, cooling down within 5 h) and forms grey-black, plate-like crystals with a metal- lic lustre. It is extremely sensitive to air and moisture.
Discussion
BaMg2Li2Si2 and BaMg2Li2Ge2 are isotypic and crystallize with a new structure type. These compounds are two new members of the growing family of the quaternary phases Ba/Mg/Li/X (X = Si and Ge) [ 1-5]. The structure of BaMg2Li2X2 (X = Si and Ge) con- tains only isolated X anions. With respect to a formal charge transfer, the compounds are described by the formulation (Ba2+)(Mg2+)2(Li+)2(X4~)2, the latter carry a formal charge of q =
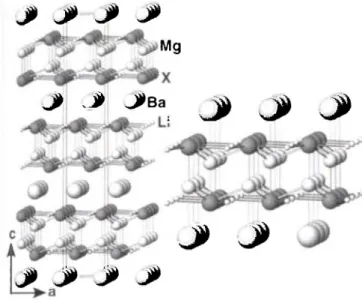
-4, which is expected according to the Zintl-Klemm concept. As shown in the figure (left: structure of BaMg2Li2X2, X = Si and Ge; right: Mg/X six ring double layer), characteristic for this structure are six ring double layers of Mg and X atoms. The latter are coordinated in an umbrella like manner by four magnesium atoms while the Mg atoms are tetrahedrally coordinated by four X atoms. These double layers are separated by layers of barium at- oms. Such structural element is already known from the crystal structure of CaAl2Si2 [6]. In fact, BaMg2Li2X2 is valence isoelectronic to CaAl2Si2. The additional lithium atoms are lo- cated in the six ring double layers in such a way that an additional planar six ring net of Li and X is formed. Due to the presence of the lithium atoms, the relative position of the six ring double layer and the layers of the alkaline earth metal atoms slightly differs from that in CaAl2Si2. In the latter, the calcium sites are always above and below the center of three silicon atoms. Since in the structure of BaMg2Li2X2 these centers are occupied by lithium, the barium atoms are located above and below the centers of the planar six ring nets of X and Li. Thus, the periodicity in the c di- rection enfolds 3 six ring double layers instead of only one found in the structure of CaAl2Si2. Interestingly, BaMg2Li2Si2 and BaMg2Li2Ge2 are isopointal with Lii4Si6 [7] and LinGeö [8].
1. Barium dimagnesium dilithium disilicide, BaMg2Li2Si2
Table 1. Data collection and handling.
Crystal: dark silver bar, size 0.11 χ 0.15 χ 0.23 mm Wavelength: Mo Ka radiation (0.71073 A)
μ: 67.27 cm"1
Diffractometer, scan mode: Stoe STADI4, ω
2ömax: 69.96°
WAA/Jmeasured, N(hkl)unique: 1427,311
Criterion for 70bs, N(hkl)g: /obs > 2 o(70bsj, 311 N(param)n fined: 13
Programs: SHELXS-97 [9], SHELXL-97 [10]
ATOMS [11]
* Correspondence author (e-mail: nesper@inorg.chem.ethz.ch)
5 0 4 BaLi2Mg2Si2 and BaLi2Mg2Ge2
Table 2. Atomic coordinates and displacement parameters (in Ä2)
Atom Site X y c Uu U22 U33 U12 uti U-.y
Ba 3 b 0 0 0 0.01553(7) Uu 0.01146(8) Uu/2 0 0
Mg 6c 0 0 0.86439(3) 0.0138(2) Uu 0.0129(3) t/ii/2 0 0
Li 6c 0 0 0.4294(2) 0.0148(9) Uu 0.037(2) Uu/2 0 0
Si 6c 0 0 0.75790(2) 0.0098(1) U\ 1 0.0118(2) Uu/2 0 0
2. B a r i u m d i m a g n e s i u m d i l i t hi u m d i g e r m a n i d e , BaLl2MgzGe2
Table 3. Data collection and handling.
Crystal:
Wavelength:
μ:
Diffractometer, scan mode:
29ma*:
N(hkl)measured. N(htt)unique:
Criterion for /obs, N(hkl)g.:
N(param)refined:
Programs:
black plate, size 0.10 χ 0.20 χ 0.25 mm Mo Ka radiation (0.71073 A) 154.48 cm"1
Stoe STADI4, ω/θ 59.84°
1300,218 /obs > 2 a(lobs), 212 13
SHELXS-97 [9], SHELXL-97 [10]
ATOMS [11]
Table 4. Atomic coordinates and displacement parameters (in Ä2).
Atom Site X y ζ Uu U22 t/33 U12 Un U11,
Ba 3a 0 0 0 0.0210(3) Uu 0.0140(3) Uu/2 0 0
Mg 6c 0 0 0.86435(9) 0.0201(6) U11 0.0161(8) Uu/2 0 0
Li 6c 0 0 0.4296(5) 0.019(3) Uu 0.036(6) Uu/2 0 0
Ge 6c 0 0 0.75706(2) 0.0148(3) Uu 0.0144(3) Uu/2 0 0
Acknowledgment. This work was supported by the Swiss National Founda- tion.
R e f e r e n c e s
1. Wengert, S.; Nesper, R.: Topological Relationships and Building Blocks in Zintl Phases of the Composition Ba^+i (Mg,Li)2jiSi2/t+i · Inorg. Chem. 39 (2000) 2861-2865.
2. Zürcher, F.; Nesper, R.: Crystal structure of hexabarium pentamagnesium trilithium dodecagermanide, Ba6Mg4.9Li3.iGei2. Z. Kristallogr. NCS 216 (2001) 505-506.
3. Wengert, S.; Nesper, R.: BaöMgiosLii j S i u , die erste Verbindung mit drei verschiedenen Zintl-Anionen. Z. Anorg. Allg. Chem. 626 (2000) 246-252.
4. Wengert, S.: Experimentelle und Theoretische Lösungsansätze zu Grundlegenden Problemen in Zintlverbindungen. Dissertation No. 12070.
ΕΤΗ Zürich, Switzerland 1997.
5. Zürcher, F.: Syntheses, Structures, and Properties of Zintl Phases Formed by the Tetrel Elements and the Alkaline-Earth Metals. Dissertation No.
12546. ΕΤΗ Zürich, Switzerland 1998.
6. Gladyshevskii, Ε. I.; Kripyakevich, P. I.; Bodak, Ο. I.: Crystal Structure of CaAl2Si2 and its Analogs. Ukr. Fiz. Zh. 12 (1967) 447-452.
7. Grüttner, Α.: Über das System Lithium-Germanium und die Bildung metastabiler Germanium-Modifikationen aus Li-Germaniden. Disserta- tion. University of Stuttgart, Germany 1982.
8. von Schnering, H. G.; Nesper, R.; Tebbe, K.-F.; Curda, J.: Structure and Properties of Lithium Silicide LiuSii (LijjjSi), the Purple Phase in the Lithium-Silicon System. Z. Metallkunde. 71 (1980) 357-363.
9. Sheldrick, G. M.: SHELXS-97. Program for the solution of crystal struc- tures. University of Göttingen, Germany 1997.
10. Sheldrick, G. M.: SHELXL-97. Program for refining crystal structures.
University of Göttingen, Germany 1997.
11.Dowty, E.: ATOMS Computer Program for Displaying Atomic Struc- tures. Shape Software, Version 3.1 for Windows, 1995.