Ζ. Kristallogr. NCS 214 (1999) 4 4 5 ^ 4 6 445
© by Oldenbourg Wissenschaftsverlag, München
Crystal structure of dilithium (nitridolithiate/manganate(I)), Li
2[(Lii_
xMn
x)N], χ = 0.73
J. Klatyk1 and R. Kniep*·1·11
I Max-Planek-Institut für Chemische Physik fester Stoffe, Pirnaer Landstr. 176, D-01257 Dresden, Germany
II Technische Universität Darmstadt, Eduard-Zintl-Institut, Hochschulstraße 10, D-64289 Darmstadt, Germany Received May 20, 1999, transferred to 2nd update of database ICSD in 1999, CSD-No. 409444
Abstract
Li2.27Mno.73N, hexagonal, P6/mmm (No. 191), a = 3.7263(4) Ä, c = 3.8281(4) Ä, V= 46.0 Ä3, Z = 1, R&(F) = 0.025,
wR(F2) = 0.063, Τ = 293 Κ.
Source of material
Single crystals of I^IXLii-jMnViN] (x = 0.73) were obtained by thermal treatment of mixtures of Li7[MnN4] [ 1 ] and Li with molar ratios of 1:1 in Ta crucibles under Ar (1 atm). The mixtures were first heated to 523 Κ (1.3 K/min, 12h) and then heated to 1173K (3 K/min). After reaching the maximum temperature the reaction products were cooled down (3 K/min) to ambient temperature.
Discussion
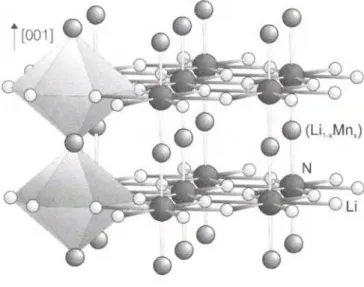
Li2[(Lio.27Mno.73)N] is a member of a substitution series Li2[(Lii-xMn'x)N] which crystallizes in the Li2[LiN] structure type [2], This general type of substitution series is already known, since the early work of Robert Juza et al. [3], who first reported the existence of ternary compounds Li2[(Lii_/TEI.t)N] (TE = Co, Ni, Cu) from X-ray powder investigations. Reinvestigations in these systems [4, 5], based on single crystal data confirmed the early observation, that the bond lenghts (Lii-jTE^-N decrease with increasing χ parameter. In case of the title compound the bond length (Lii-xMnJ-N (x = 0.73) within the infinite chains is shortened from 193.8(1) pm in Li2[LiN] (x = 0) [2] to 191.4(2) pm. It is interesting to note that the decrease of the bond lengths of (Lii-jMnJ-N with increasing χ parameter is much smaller compared with the isotypic phases Li2[(Lii-xTE'x)N]
(TE1 = Fe [6], Co, Ni, Cu [3-5]).
Table 1. Data collection and handling.
Crystal: brass plate, size 0.2 χ 0.2 χ 0.4 mm Wavelength: Mo Ka radiation (0.71070 A)
μ: 48.43 cm"1
Diffractometer, scan mode: Siemens P4, ω/2θ
28max: 59.6°
N(hkl)measured, N(hkl)miquc: 257,44
C r i t e r i o n f o r /0bs, N(hkl)gt: /obs > 2 cf/obs), 44
N(param),er,ned'· 8
Programs: SHELXL-97 [7], DIAMOND [8]
Table 2. Atomic coordinates and displacement parameters (in Ä2).
Atom Site X y ζ t/ll U22 U33 Un Un Un
Mn lb 0.73(2) 0 0 1/2 0.0305(6) Un 0.0081(6) 0.0153(3) 0 0
Li(l) lb 0.27 0 0 1/2 0.0305 Uu 0.0081 0.0153 0 0
Ν la 0 0 0 0.019(2) U\i 0.009(2) 0.0094(8) 0 0
Li(2) 2c 1/3 2/3 0 0.047(4) Uu 0.040(6) 0.024(2) 0 0
* Correspondence author (e-mail: klatyk@cpfs.mpg.de)
446 Dilithium (nitridolithiate/manganate(I))
Acknowledgment. We thank the Deutsche Forschungsgemeinschaft (Schwerpunktprogramm "Reaktivität von Festkörpern") for supporting this work.
References
1. Juza, R.; Anschütz, E.; Puff, H.: Die Struktur von L17VN4 und Li7MnN4.
Angew. Chem. 71(1959) 161.
2. Rabenau, Α.; Schulz, Η.: Re-Evaluation of the Lithium Nitride Structure.
J. Less-Common Met. 50 (1976) 155-159.
3. Sachsze, W., Juza, R.: Über Mischkristalle der Zusammensetzung (Li,Co)3N, (Li,Ni)3N und (Li,Cu)3N. Z. Anorg. Allg. Chem. 259 (1948) 278-290.
4. Gudat, Α.: Ternäre und quaternäre Nitride und Nitridometallate in Systemen Lithium-Erdalkalimetall-Übergangsmetall-Stickstoff. Disser- tation, Universität Düsseldorf, Germany 1990.
5. Höhn, P.: Temäre und quaternäre Nitridometallate: Verbindungen in den Systemen Lithium-Erdalkalimetall-Übergangsmetall-Stickstoff.
(Übergangsmetall = Ta, Mo, W, Fe, Co). Dissertation, TH Darmstadt, Germany 1993.
6. Klatyk, J.; Kniep, R.: Crystal structure of dilithium (nitrido- lithiate/ferrate), Li2[Lii-IFei)N], λ = 0.63. Ζ. Kristallogr. NCS 214 (1999) 447-448.
7. Sheldrick, G. M.: SHELXL-97, a program for refining crystal structures.
University of Göttingen, Germany 1997.
8. Brandenburg, K.: DIAMOND (Version 2.1a). Crystal Impact GbR, Germany 1996-1999.