Research Collection
Doctoral Thesis
A marriage made in soil - quantifying bacterial life in soil
hotspots using individual-based and metabolic network modeling
Author(s):
Borer, Benedict Publication Date:
2020-08
Permanent Link:
https://doi.org/10.3929/ethz-b-000431617
Rights / License:
In Copyright - Non-Commercial Use Permitted
This page was generated automatically upon download from the ETH Zurich Research Collection. For more information please consult the Terms of use.
ETH Library
A marriage made in soil
– quantifying bacterial life in soil hotspots using individual-based and metabolic network modeling
Benedict Borer
ETH Diss. No: 26658
DISS. ETH NO. 26658
A marriage made in soil
– quantifying bacterial life in soil hotspots using individual-based and metabolic network modeling
A thesis submitted to attain the degree of DOCTOR OF SCIENCES of ETH ZURICH
(Dr. sc. ETH Zurich)
Presented by
BENEDICT ALOIS BORER
MSc Hydrology, Imperial College London born on 06.10.1989
citizen of Büsserach, SO
accepted on the recommendation of Prof. Dr. Dani Or
Prof. Dr. Martin Ackermann Dr. Jan-Ulrich Kreft
2020
Abstract
Soil microorganisms are at the core of many global biogeochemical and ecological processes, such as the carbon and nitrogen cycles and associated greenhouse gas emissions. For scale, soil prokaryotes produce an amount of carbon dioxide equivalent to that produced by all of the oceans combined, and contribute 60% of the global NOx emissions. Evidence suggests that bacterial-driven biogeochemical functions rely on spatially organized microbial communities whose activity is mediated by the aqueous phase configuration and a highly heterogeneous nutrient landscape. Microbial cells inhabit only a fraction of the available soil surface and communities often congregate to form dense microbial hotspots around patchy resources with disproportional local metabolic activity that sustains important biogeochemical fluxes. Despite the apparent importance of the localized activity in soil, we lack the observational capabilities and a mechanistic understanding to understand how these hotspots emerge, persist and function. This dissertation employs a combination of experimental and computational approaches to tackle visualization and quantification of processes shaping microbial community structure and spatial organization at the pore scale. Providing a mechanistic understanding of bacterial life at the pore scale paves the way to predict greenhouse gas emissions at the pedon scale – and ultimately to integrate to global biogeochemical cycles.
The dissertation begins with a direct visualization of spatially self-organizing synthetic microbial communities in response to commonly reported nutrient and oxygen counter-gradients at the pore scale. We developed glass-etched pore networks retaining salient features of soil whilst permitting visualization of fluorescently tagged bacterial species with different metabolic capabilities. The spatial structure provided by the pore network promoted a segregation of an initially mixed community mediated by carbon and oxygen counter-gradients and enabled the coexistence of the two species (which is in contrast to observations in well-mixed systems).
We gained new insights into the underlying nutrient landscape of this experimental system by using a novel mathematical framework that combined an individual-based representation of bacterial cells with a physical representation of their habitat as angular pore networks. The diverse metabolic capabilities of soil bacteria were captured using genome-scale metabolic networks, which provided a natural mechanism for the switching between aerobic and anaerobic growth strategies frequently encountered in soil due to episodic wetting events and associated diffusion limitations. Spatial segregation of the two subpopulations was maintained through cross-feeding anaerobically produced intermediate metabolites supporting aerobic growth at the hotspot periphery. This improved our understanding of how bacterial species engage in trophic interactions mediated by the spatial
structure of the habitat, diffusion characteristics determined by the aqueous phase configuration, and bacterial metabolic capabilities.
The importance of metabolic cross-feeding interactions in soil food webs raises the question of whether we can harness genome-scale metabolic networks to predict trophic interactions among multi-species communities and their stable composition (at steady state). We used an environmentally linked metabolic network model to predict the community structure (species composition) that emerges when growing on various carbon sources entering the metabolism through different pathways. Accompanying laboratory experiments using viable cell counts and qPCR provided the opportunity to validate our approach of linking the ever-growing wealth of genetic data (here in the form of metabolic networks) to microbial community structure and ultimately function.
In contrast to the experimental system described above, where individual cells were at liberty to relocate rapidly, microbial life in soil is predominantly associated with sessile assemblages. Hence, initial spatial positioning, steep nutrient gradients and localized growth conditions play a fundamental role in shaping the makeup of such communities. We investigated emerging spatial patterns in the bacterial community depending on trophic interactions and provided a unified mechanism for how relative growth-rate differences rooted in the underlying nutrient landscape mediate emerging patterns, with far-reaching consequences for individual cell lineage proliferation and ecological function. We included further structural heterogeneities in the habitat to study the influence of obstacles on the consequences for population genetics and community function.
The penultimate chapter of the dissertation provides an overview of the vastly broad distribution of soil bacterial growth rates and associated generation times. The microscale architecture of soil with localized and steep resource gradients supports formation of microbiological hotspots of high bacterial cell growth rates adjacent to “cold spots” with seemingly dormant cells experiencing no net growth.
The coexistence of cells of the same species in close proximity to hundreds of microns that represent vastly different “ages” and generations within the same soil volume, and which may occasionally reconnect upon soil wetting, provides unique opportunities for unparalleled genetic memory and interactions across “ages” not found in any other compartment of the biosphere. The consequences of such a large age distribution in the soil and the ramifications for trait conservation and genetic memory are discussed.
Overall, the main theme of this dissertation is to harness the power of mathematical models that incorporate soil physical heterogeneity and embrace details of bacterial metabolic versatility in the form of genome-scale metabolic networks to advance our understanding of how physical, chemical and biotic factors shape the spatial self-organization and functioning of microbial communities in soil.
Constraining such mathematical frameworks with laboratory experiments and environmental measurements is of paramount importance, given that soil microbiota are considered key drivers in global biogeochemical cycles. We are at a pivotal time in soil microbial ecology, when advances in experimental techniques enable quantification of processes and fluxes at an ever-increasing resolution – down to single communities, populations and even individual cells in situ. In parallel, computational capabilities are developing at an equivalent rate, providing novel opportunities through innovative mathematical concepts and large-scale computation facilities available to researchers. Unifying such experimental and computational efforts enables scenarios where microbial processes in global-scale land-surface models will not be driven by simplified empirical relations – but rather by mechanistic and physically based predictions of microbially driven fluxes at all scales.
Zusammenfassung
Bodenmikroorganismen stehen im Mittelpunkt vieler globaler biogeochemischer und ökologischer Prozesse wie des Kohlenstoff- und Stickstoffkreislaufs und der damit verbundenen Treibhausgasemissionen. Zum Vergleich: Prokaryonten im Boden produzieren eine äquivalente Menge Kohlenstoffdioxid wie die Summe aller Ozeane und verursachen 60% der globalen NOx-Emissionen. Es gibt Hinweise darauf, dass bakteriell beeinflusste biogeochemische Funktionen auf räumlich höchst organisierte Gemeinschaften zurückzuführen sind, deren Aktivität durch die Konfiguration der wässrigen Phase und eine heterogene Nährstofflandschaft beeinflusst wird. Mikrobielle Zellen bewohnen nur einen Bruchteil der verfügbaren Bodenoberfläche, und die Individuen aggregieren oft zu dichten mikrobiellen Hotspots mit einer unverhältnismäßig hohen lokalen Stoffwechselaktivität, welche wichtige biogeochemische Flüsse aufrechterhält. Trotz der offensichtlichen Bedeutung der lokalisierten Aktivität im Boden fehlen uns Beobachtungsmöglichkeiten und ein mechanistisches Verständnis dafür, wie diese Hotspots entstehen, fortbestehen und funktionieren. In dieser Arbeit verwenden wir eine Kombination aus experimentellen und mathematischen Ansätzen, um die Visualisierung und Quantifizierung mikrobieller Prozesse zu untersuchen, welche die Zusammensetzung und räumliche Organisation von bakteriellen Gemeinschaften im Porenmassstab grundlegend beeinflussen. Die Bereitstellung eines mechanistischen Verständnisses des bakteriellen Lebens im Porenmassstab ebnet den Weg zur Vorhersage von Treibhausgas-Emissionen auf der Pedon- Skala - und letztlich zur Integration in globale biogeochemische Kreisläufe.
Die Dissertation beginnt mit einer direkten Visualisierung von sich räumlich selbstorganisierenden synthetischen mikrobiellen Gemeinschaften, gelenkt durch häufig beobachtete Gegengradienten von Kohlenstoff und Sauerstoff in Hotspots. Wir haben glasgeätzte Porennetzwerke entwickelt, welche manche grundlegenden Merkmale des Bodens beibehalten und gleichzeitig die Visualisierung von fluoreszenzmarkierten Bakterienarten mit unterschiedlichen metabolischen Fähigkeiten ermöglichen.
Die durch das Porennetzwerk bereitgestellte räumliche Struktur und Gegengradienten fördern die Aufspaltung einer zunächst gemischten Gemeinschaft und ermöglichen die Koexistenz der beiden Arten (was in gut gemischten Systemen nicht der Fall ist).
Wir gewannen neue Einblicke in die Verteilung der Nährstoffe innerhalb des experimentellen Systems, indem wir ein mathematisches Konstrukt verwendeten, welches einen Individuen-basierten Ansatz zur Beschreibung der Bakterienzellen mit einer physikalischen Darstellung ihres Habitats als Winkel- Porennetzwerke kombiniert. Die vielfältigen Stoffwechselfähigkeiten der Bodenbakterien werden mit Hilfe metabolischer Netzwerke (basierend auf ganzen Genomen) erfasst, die einen natürlichen Mechanismus für den Wechsel zwischen aeroben und anaeroben Wachstumsstrategien bieten, welche
im Boden aufgrund episodischer Benetzung und damit verbundener Diffusionsbeschränkungen häufig vorkommen. Die räumliche Trennung der beiden Subpopulationen wird durch die gegenseitige Syntrophie von anaerob produzierten Metaboliten aufrechterhalten, die das aerobe Wachstum an der Peripherie des Hotspots unterstützen. Dies verbesserte unser Verständnis, wie trophische Interaktionen zwischen Bakterien in Hotspots entstehen, abhängig von der räumliche Struktur des Habitats, der Diffusionseigenschaften (welche durch die Konfiguration der wässrigen Phase bestimmt werden) und der bakteriellen Stoffwechselfähigkeiten.
Die Bedeutung der uni- oder bilateralen syntrophischen Interaktionen in mikrobiellen Nahrungsnetzen des Bodens wirft die Frage auf, ob wir mittels metabolischer Netzwerke die stabile Zusammensetzung mikrobieller Gemeinschaften vorhersagen können. Wir verwenden ein dynamisches metabolisches Netzwerkmodell mit externen Metaboliten, um die entstehende Artenzusammensetzung beim Wachstum auf diversen Kohlenstoffquellen - die über verschiedene Wege in den Stoffwechsel gelangen - vorherzusagen. Begleitende Laborexperimente ermöglichen die Validierung unseres Ansatzes, die ständig wachsende Menge genetischer Daten (hier in Form von metabolischen Netzwerken) mit der Struktur und letztlich der Funktion der mikrobiellen Gemeinschaften zu verknüpfen.
Im Gegensatz zu dem oben beschriebenen experimentellen System, bei dem die einzelnen Zellen in ihrer Mobilität nicht eingeschränkt sind, wird das mikrobielle Leben im Boden überwiegend mit sessilen Kolonien in Verbindung gebracht. Daher spielen die räumliche Positionierung einzelner Zellen in der Kolonie, die steilen Nährstoffgradienten und die damit verbundenen lokalisierten Wachstumsbedingungen eine grundlegende Rolle bei der entstehenden Zusammensetzung solcher Gemeinschaften. Wir untersuchen die Entstehung räumlicher Muster der bakteriellen Gemeinschaft in Abhängigkeit von trophischen Interaktionen und liefern einen einheitlichen Mechanismus, wie relative Wachstumsunterschiede (welche in der zugrundeliegenden Nährstofflandschaft verwurzelt sind) die Entstehung von bakteriellen Mustern ermöglichen, mit weitreichenden Folgen für die Abstammungslinie von individuellen Zellen und die ökologische Funktion. Wir erhöhen die Heterogenität des bakteriellen Habitats, um den Einfluss von Hindernissen auf die genetischen Folgen der Population und die Funktion der Gemeinschaft zu untersuchen.
Acknowledgements
Soil is a notoriously heterogeneous and dynamic system – echoing the feelings I have had about this thesis over the years. Having spent a considerable time within the STEP group and the MicroscapesX project, I had the pleasure of working with numerous people – all of whom influenced me in some way during my PhD and whom I hope to recognize in these acknowledgements, although this list is not exhaustive.
First of all, I am extremely grateful for the opportunity to conduct my PhD research in the group of Dani Or. His patience and guidance during my journey through the PhD are more adequately characterized by the German word “Doktorvater” than simply “supervisor”. His motivation and dedication to understanding processes in soil from a mechanistic perspective are inspiring, and certainly influenced my decision to pursue further research at the interface of biochemistry and microbial ecology. Next, I would like to thank the external members of my PhD committee, Martin Ackermann and Jan-Ulrich Kreft, both of whom had a significant influence on my career by introducing me to the fields of microbiology and individual-based modeling, respectively, which are the two main thematic pillars of this thesis.
During my time in the STEP group, I made many new friends and worked with many people who contributed to an unforgettable journey. A special mention needs to be made of the SoilBugs microbiology group (Robin, Hannah, Davide, Sämi, Samy, Ali, Minsu, Gang and Liliana) and friends/colleagues working on different aspects of soil physics (Peter, Andreas, Sabine, Bassem, Surya, Frouke, Linfeng, Erfan, Milad, Leonardo, Stan, … I am sure that there are many more!) who shaped both my thinking and research. Finally, I had the privilege of working with three incredible technicians (Dani, Hans and Simon) who designed solutions for every crazy experimental idea – and their solutions always worked! Further, I would like to thank Kayla Bolsinger for illustrating the thesis cover (putting up with my obsession with bacterial personification). I am enormously grateful to my mother, who spent hours editing my manuscripts and especially the thesis – including strict grammar surveillance of my writing!
Last but certainly not least, I would like to thank my parents (Nikki and George), my sister (Jess) and best friend (Alexis), who have supported me every day of my life – especially during stressful and demanding times. I hugely appreciate the solid foundation that you provide, giving me both the opportunity and motivation to pursue any objective in life – a liberty that I will never take for granted.
July 2020
TABLE OF CONTENTS
CHAPTER 1 ... 1
Thesis introduction ... 1
1.1 Bacterial abundance and distribution in soil ... 2
1.2 Capturing soil microhydrology and microbial life in mathematical frameworks ... 4
1.3 Dynamic modeling of soil multispecies microbial communities ... 6
1.4 Emerging spatial patterns in sessile bacterial range expansion ... 7
1.5 Generation time distribution in soil ... 8
1.6 References ... 9
CHAPTER 2 ... 13
Spatial organization of bacterial populations in response to oxygen and carbon counter-gradients in pore networks ... 13
2.1 Abstract ... 14
2.2 Introduction ... 15
2.3 Results ... 17
2.4 Discussion ... 25
2.5 Materials and methods ... 29
2.6 Acknowledgements ... 35
2.7 References ... 36
2.8 Supplementary material ... 40
CHAPTER 3 ... 45
Modeling metabolic networks of individual bacterial agents in heterogeneous and dynamic soil habitats (IndiMeSH) ... 45
3.1 Abstract ... 46
3.2 Introduction ... 47
3.3 Methods ... 49
3.4 Results ... 59
3.5 Discussion ... 65
3.6 References ... 69
3.7 Supplementary material ... 72
CHAPTER 4 ... 77
Primary carbon sources and self-induced metabolic landscapes shape community structure in soil bacterial hotspots ... 77
4.1 Abstract ... 78
4.2 Introduction ... 79
4.3 Results ... 82
4.4 Discussion ... 87
4.5 Materials and methods ... 91
4.6 References ... 95
4.7 Supplementary material ... 98
CHAPTER 5 ... 101
Spatial organization in microbial range expansion emerging from trophic dependencies and successful lineages ... 101
5.1 Abstract ... 102
5.2 Introduction ... 103
5.3 Results ... 105
5.4 Discussion ... 113
5.5 Materials and methods ... 117
5.6 References ... 121
5.7 Supplementary material ... 124
CHAPTER 6 ... 127
Bacterial age distributions in soil – life cycles in hot and cold spots ... 127
6.1 Abstract ... 128
6.2 Introduction ... 129
6.3 Modeling bacterial age and cell lineages in soil ... 132
6.4 Results ... 134
6.5 Discussion ... 139
6.6 References ... 143
6.7 Supplementary material ... 146
CHAPTER 7 ... 155
Conclusions and outlook ... 155
7.1 Summary and conclusions ... 156
7.2 Outlook and future perspectives ... 159
7.3 Concluding remarks ... 161
7.4 References ... 162
APPENDIX 1 ... 163
The engineering of spatially linked microbial consortia – potential and perspectives ... 163
A1.1 Abstract ... 164
A1.2 Introduction ... 165
A1.3 Experimental consortia of spatially linked and interacting microorganisms ... 167
A1.4 Mathematical modeling for designing and assembling microbial consortia ... 171
A1.5 The assembly of misfits ... 173
A1.6 Conclusion and outlook ... 175
A1.7 References ... 176
APPENDIX 2 ... 181
Reduced gravity promotes bacterially mediated anoxic hotspots in unsaturated porous media ... 181
A2.1 Abstract ... 182
A2.2 Introduction ... 183
A2.3 Results ... 185
A2.4 Discussion ... 192
A2.5 Methods and materials ... 194
A2.6 Acknowledgements, Data and code availability, and Competing interests ... 198
A2.7 References ... 199
A2.8 ASupplementary material ... 201
CURRICULUM VITAE BENEDICT BORER ... 203
LIST OF FIGURES
Figure 1.1: Thematic overview of this dissertation. ... 3 Figure 2.1: Conceptual view of a model pore network. ... 17 Figure 2.2: Images of the micrometric pore networks with varying levels of pore connectivity. ... 18 Figure 2.3: Experimentally observed spatial segregation of bacterial populations in a micrometric
pore network. ... 20 Figure 2.4: Distribution of aerobes and anaerobes as a function of spatial positioning within the
different pore networks. ... 22 Figure 2.5: Relative abundance of the two species in the community as a function of growth
conditions and spatial structure of the environment. ... 24 Supplementary Figure 2.6: Comparison of Euclidean distance vs shortest paths within the network
topologies. ... 40 Supplementary Figure 2.7: Visualization of oxygen concentrations at the central and peripheral ports
of the pore network. ... 40 Supplementary Figure 2.8: Additional simulations to elucidate the importance of factors governing
spatial self-organization. ... 41 Supplementary Figure 2.9: Simulations to elucidate the interaction between network connectivity
and relative abundance of the two species. ... 42 Supplementary Figure 2.10: Mathematical model predictions of bacterial spatial distribution and
corresponding oxygen and citrate concentration fields in the pore network. ... 43 Supplementary Figure 2.11: Comparison of spatial segregation and oxygen concentration profile
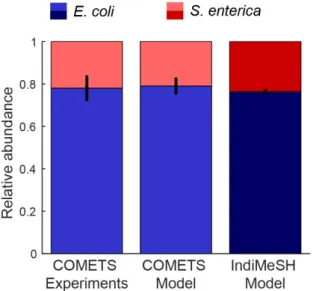
between two model versions. ... 44 Figure 3.1: Conceptual image of IndiMeSH model components and methods. ... 50 Figure 3.2: Relative abundance of E. coli and S. enterica in the IndiMeSH simulations and original
COMETS simulations and experimental results. ... 60 Figure 3.3: Distribution of obligate aerobic P. putida (cyan) and facultative anaerobic P. veronii
(magenta) along the carbon-oxygen counter gradient from the central port to the peripheral ports. ... 61 Figure 3.4: Response of bacterial population to a shift in boundary conditions represented as glucose
perfusion into a soil aggregate cross-section. ... 62 Figure 3.5: Strategic analysis of pore size distribution and hydration conditions. ... 63 Supplementary Figure 3.6: Geometry definition for angular pore cross-sections... 72 Supplementary Figure 3.7: Habitat geometry, boundary conditions and inoculation location for all
simulation scenarios. ... 72 Supplementary Figure 3.8: Comparison of COMETS and IndiMeSH motility predictions. ... 73 Supplementary Figure 3.9: Comparison of E. coli GEM and rGEM in varying environmental conditions.
... 74 Figure 4.1: Bottom-up modeling approach of integrating genomic information into mechanistic
modeling of bacterial life to predict ecosystem properties and function. ... 81 Figure 4.2: Prediction of community composition on various carbon sources with experimental
validation. ... 83 Figure 4.3: Growth of the four species bacterial community in a spatiotemporal setting. ... 86 Supplementary Figure 4.4: Prediction of community composition on 30 carbon sources with
experimental validation. ... 98
Figure 5.1: Spatial patterns emerging from different trophic interactions among members of a
synthetic bacterial community grown on agar surfaces. ... 106 Figure 5.2: The underlying chemical landscape governs local growth rates of the bacterial colony. 108 Figure 5.3: Trophic interactions alter the reproductive success of individual cells. ... 110 Figure 5.4: Simulated two-species bacterial colony expansion into domains with different spatial solid
structures – impacts on lineage proliferation with potential ecological and evolutionary
consequences. ... 112 Supplementary Figure 5.5: Importance of bacterial shoving and initial spatial positioning for pattern
formation. ... 124 Supplementary Figure 5.6: Inclusion of stochastic variations in particle size simulations of structured
habitats (with different obstacle spacing)... 125 Figure 6.1: Distribution of bacterial generation times in topsoil and the rhizosphere. ... 130 Figure 6.2: Bacterial population demographics shaped by diffusion and dispersal limitations around
soil hotspot as affected by hydration conditions. ... 133 Figure 6.3: Variations in simulated bacterial population sizes and generation time distributions with
hydration conditions. ... 135 Figure 6.4: Characteristics of dominant (highest reproductive success) and rare lineages vary with
hydration conditions. ... 138 Supplementary Figure 6.5: Fitting (exponentially truncated) power law distributions to IndiMeSH
simulation data. ... 146 Supplementary Figure 6.6: Fitting (exponentially truncated) power law distributions to extrapolated
IndiMeSH simulation data. ... 147 Supplementary Figure 6.7: Mean growth rates of individual lineages through time for two contrasting hydration conditions. ... 148 Supplementary Figure 6.8: Cell dispersal and diffusion regime determine bacterial generation time
distribution. ... 149 Figure A1.1: From monocultures to spatially linked microbial consortia. ... 166 Figure A1.2: Functional elements for the culture of spatially linked microbial consortia. ... 170 Figure A1.3: Design of a glass beads column with thermal gradient spatially segregating a microbial
consortium for (anaerobic) methane production from cellulose. ... 174 Figure A2.1: Fully assembled glass pore network and conceptual image... 186 Figure A2.2: Comparison of the water table, capillary fringe and extent of anoxic hotspots depending
on the gravitational conditions. ... 187 Figure A2.3: Temporal dynamics of oxygen saturation in the presence and absence of bacteria. .... 188 Figure A2.4: Changes in the anoxic conditions and wetted area with changing gravity conditions. .. 189 Figure A2.5: Mathematical model and simulation results investigating the effect of bacterial density
on oxygen dynamics. ... 191 Supplementary Figure A2.6: Experimental conditions within pore networks for each set of parabolas.
... 201 Supplementary Figure A2.7: Comparison of calibration images with an inoculated network image. 202
LIST OF TABLES
Table 2.1: Parameters used in the mathematical model and their source reference. ... 34
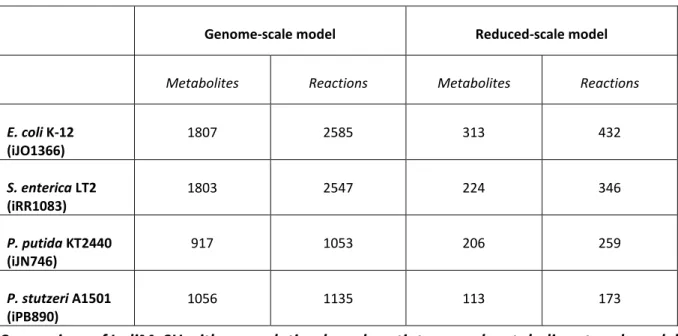
Table 3.1: Summary of genome-scale and reduced-scale metabolic network dimensions for all species. ... 55
Table 3.2: Evaluation of metabolic network reduction and comparability to genome-scale metabolic networks. ... 59
Supplementary Table 3.3: Summary of maximum uptake rates imposed for all metabolic networks and simulation scenarios. ... 75
Supplementary Table 3.4: List of parameters that can be set by the model user. ... 76
Table 4.1: Genome-scale reconstructions used in this study. ... 91
Table 4.2: qPCR primers used in this study. ... 92
Supplementary Table 4.3: Single species OD and predicted FBA doubling time for 15 carbon sources. ... 99
Supplementary Table 4.4: Modifications to curated metabolic network based on observed growth capabilities. ... 99
Supplementary Table 4.5: Maximum uptake rates for each metabolite. ... 100
Table 5.1: List of modeling parameters and references. ... 120
Supplementary Table 5.2: Quantification of total number of peripheral lineages [#], persistent consumer branches [#] and biomass [10-8 kg] for competition (CT), weak mutualism (WM) and strong mutualism (SM) in all simulated habitat scenarios. ... 126
Supplementary Table 6.1: Parameters used in the mathematical model (IndiMeSH). ... 151
Supplementary Table 6.2: Simulated mean cell age [days] as a function of matric potential for different cumulative biomass cutoff threshold. ... 152
1
Chapter 1
Thesis introduction
2
1.1 Bacterial abundance and distribution in soil
Soil is the richest compartment of the biosphere, harboring unparalleled biodiversity containing up to four million species in a ton of soil (compared to two million species in all oceans combined)1 and a prokaryote density 200,000 times higher than found in the oceans2. Despite their overall high abundance, soil prokaryotes are sparsely distributed resulting in dense microbial assemblages (termed hotspots3) often coinciding with nutrient and carbon rich environments such as the rhizosphere4,5, detritusphere6, biopores7–9 or soil aggregates10,11. Intense microbial activity within these hotspots gives rise to anoxic microsites, contributing disproportionally to the total microbial activity. Evidence shows that microbial anoxic hotspots comprising 1% of the soil volume can account for up to 90% of the total nitrous oxide emissions12, and are responsible for 60% and 70% of the global methane and NOx emissions, respectively13. Counterintuitively, anoxic microsites readily occur in unsaturated, well- aerated soils14 due to oxygen diffusion limitations. A prime factor shaping the diffusive landscape in soil is the aqueous phase architecture, posing a barrier for gaseous diffusion in saturated pore spaces (diffusion coefficient in water approximately 1000 times lower compared to air) thus facilitating the generation of anoxic microsites. As soil becomes partially unsaturated, aqueous diffusion is limited to thin water films retained in crevices between solid soil particles behind liquid-air interfaces15,16. The interplay of gaseous and aqueous diffusion limitations results in an optimal hydration condition for microbial activity in conditions where gaseous and aqueous diffusion is optimal for cell growth17,18. In addition to shaping the habitat of bacterial cells through diffusion limitations, the aqueous phase architecture also affects bacterial life directly through dispersal limitations, where thin water films essentially immobilize bacterial cells through pinning forces19,20. Nevertheless, an increasing body of evidence supports the notion that microbial distributions in soil are not random but consist of highly organized bacterial communities21–23 with far reaching consequences for their ecological functioning24. In addition to the complexity of scales and heterogeneity outlined above, unveiling the spatial distribution of bacteria in soil at the relevant scale is nigh impossible due to restrictions for direct visualization imposed by soil opacity25. These limitations have been circumvented by thin slicing and subsequent microscopy23, however this approach restricts identification of different bacterial species.
In Chapter 2, we use an experimental system comprised of glass-etched pore networks with controllable nutrient boundary conditions to directly observe the spatial distribution of a fluorescently tagged microbial community in response to carbon and oxygen counter-gradients and therefore circumvent the issue of opacity encountered in natural soils26.
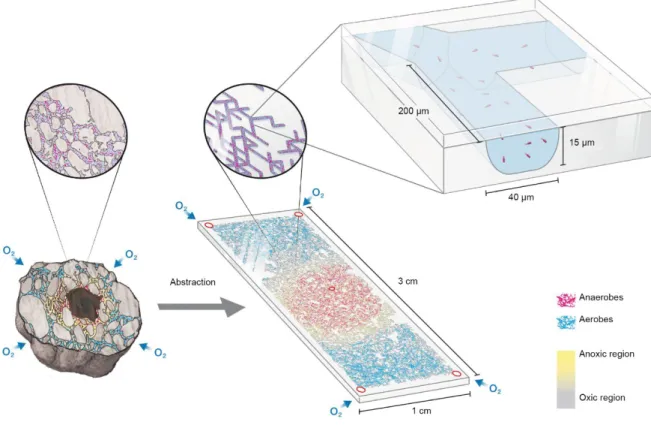
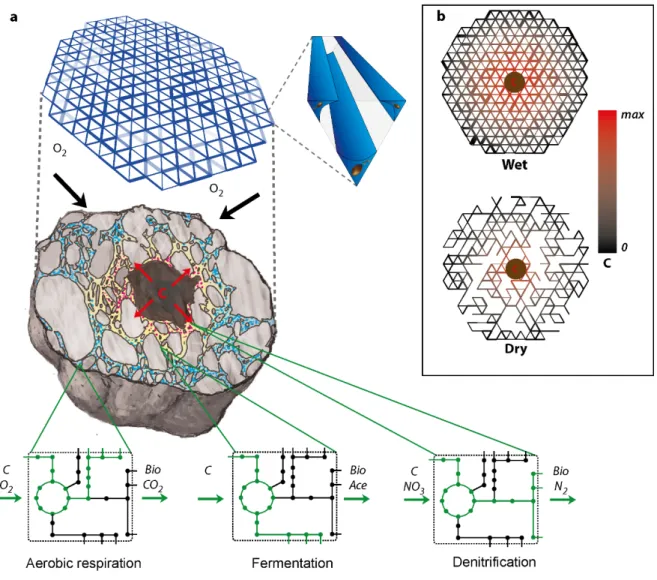
3 Figure 1.1: Thematic overview of this dissertation.
a) Simplifications and abstractions are made to manage the incredible complexity of soil and enable us to represent soil-like environments as angular pore networks and study soil bacterial hotspots. b) We used micrometric pore networks to study the spatial self-organization of synthetic communities in response to nutrient and oxygen counter-gradients (Chapter 2). c) We developed mathematical models that incorporate mechanistic and physically based representations of soil microbial habitats and details of individual bacterial cell behavior including their metabolic versatility to study bacterial life in soil (Chapter 3) and explicitly for bacterial multispecies communities (Chapter 4), with ramifications for bacterial age distributions and the “genetic reservoir” of soil (Chapter 6). d) The majority of microbial life on this planet is in the form of sessile microbial assemblages where range expansion through growth results in highly organized “bacterial cities”. e) We developed a mathematical framework that combines single cell processes with microscale nutrient diffusion to elucidate drivers of bacterial growth dynamics and emergent pattern formation with consequences for ecosystem functioning (Chapter 5). f) We explored the influence of gravity on oxygen dynamics in artificial soil bacterial hotspots mediated by the aqueous phase configuration with potential ramifications for cabin atmosphere and Martian soil remediation (Appendix 2).
4
1.2 Capturing soil microhydrology and microbial life in mathematical frameworks
Due to the inherent complexity of soil pore spaces27, representing the habitat of microbes in mathematical models adequately poses a great challenge. Recently, advances have been made by abstracting pore spaces to roughness networks28, idealised rough surfaces29 and 3D angular pore networks18,20. A key benefit of angular pores (e.g. represented by a triangular cross-section) is the physically based description of the aqueous phase configuration, where thin water films are retained in corners behind liquid-air interfaces30 allowing dual-phase occupancy of water and air mediating diffusional constraints. A detailed description of the physical and chemical environment with the capability of emerging nutrient heterogeneity and fluctuating redox conditions is of paramount importance to capturing soil microbial responses. A second important consideration is the representation of bacteria within the model. Agent-based (or individual-based) approaches have been frequently used to study microbial systems such as wastewater, phytoplankton communities, biofilms or food spoilage (an excellent review of different applications is given by Hellweger et al. 200831 and more recently in Hellweger et al. 201632). Individual-based models (IBMs) are especially suited for microbial systems as they bridge the gap between the behavior at the single cell level and emerging processes, organization and functions at the community level32 despite individual agents lacking global knowledge of system properties – a principle fundamental to good practice in individual-based modeling! In soil, this enables a direct link between physical processes such as nutrient diffusion and dispersal limitation due to liquid-air interface induced pinning forces19,20,28 with system properties such as the spatial self-organization of microbial communities18,33, trophic interactions34, greenhouse gas production35 and coexistence due to habitat fragmentation28,29. An essential component of IBMs simulating bacterial life in soil is the relationship between localized nutrient conditions and bacterial biomass synthesis, and is frequently represented by simple Monod type kinetics and stoichiometric relationships18,29,34,36–38. A pitfall of these simple kinetics is their inability to capture the facultative anaerobic behavior of cells in response to oxygen limitations in a natural way – conditions frequently experienced in soil due to episodic wetting events and diffusion limitations posed by water-filled pore spaces (an interesting approach of switching metabolism is used by Lardon et al. 201138). A method to circumvent this limitation is the use of genome-scale metabolic networks (GEMs) and constraint-based modeling to directly link localized nutrient availability with bacterial growth and consumption rates39, switching naturally from aerobic respiration to anaerobic metabolism and associated intermediate metabolite production depending on the species’ metabolic capabilities.
Since the first genome-scale reconstruction of Haemophilus influenza RD in 199940, metabolic modeling of microbial metabolism has become increasingly valuable through an ever increasing availability of genome-scale reconstructions and mathematical capabilities for analyzing the latter41.
5
As of February 2019, a total of 6239 genome-scale reconstructions have been reported, of which 5897 are bacteria, 127 archaea and 215 eukaryotes41. The large number of bacterial reconstructions is owing to semi-automated approaches such as Path2Model42, AGORA43 or CarveMe44. This is underlined by the fact that only 113 of the 5897 bacterial reconstructions have been subjected to manual curation41, of which 108 are conveniently collected in the BIGG database45. In recent years, metabolic modeling based on genome-scale metabolic networks has been implemented dynamically (dynamic flux balance analysis) and in a spatial context (spatiotemporal metabolic network modeling) for various microbial habitats (an excellent review is provided by Henson et al. 201546). Models combining a spatiotemporal setting with both a population based approach47 and individual-based approaches48,49 have been developed. A key benefit of these models are predictions of localized metabolic activity and their ability to discover putative cross-feeding relationships rather than assuming them a priori. However, these models rely on a simple representation of the physical habitat and are not suited to capture the nuanced nutrient patchiness expected in soil.
In Chapter 3, we combine a physically based description of soil pore spaces with an individual-based representation of bacterial cells, where biomass synthesis is linked to localized nutrient conditions using genome scale and reduced scale metabolic networks50. We seek model validation through comparison with a previously published population-based spatiotemporal metabolic network model, strategically investigate the importance of hydration conditions and nutrient boundary conditions, and highlight the importance of trophic interactions in a spatial context for community stability and coexistence.
6
1.3 Dynamic modeling of soil multispecies microbial communities
Recently, advances in sequencing technologies have provided insights into natural microbial communities − including the currently uncultivatable fraction51 − and are rapidly expanding the amount of genetic data available. One of the main challenges is to relate the genetic data to community structure and microbial ecosystem function52. Based on the gene-protein-reaction relationship captured in metabolic modeling approaches, spatiotemporal metabolic network models that are extended to simulate multispecies communities are a prime candidate to bridge the gap between genetic information and ecosystem functioning. There are several commonly used methods to represent communities in constraint-based modeling52,53 such as linking individual genome-scale reconstructions via the environment, compartmentalization into a large network, and the “enzyme soup” approach where a single network is created combined from all metabolic capabilities of the species present. Such models have been successfully applied to study fundamental aspects of microbial life, such as the prediction of competition/mutualism interactions54–56 or community response to nutrient modulations57; but also for more specifically bioengineering applications, such as insights into pathogenesis mechanisms58 and biomining/bioremediation59. Soil represents an interesting case for multispecies metabolic modeling because of the wide variety of carbon sources available in soil due to translocation of assimilated carbon by plants (up to 2200 kg C per year and hectar)60 or plant litter resulting in decomposition within the detritusphere61. This cocktail of carbon sources and fluctuating redox conditions prevailing in soils raises the question of whether we are capable of predicting microbial community composition and dynamics of multi-species bacterial communities using constraint-based modeling approaches.
In Chapter 4, we develop an environmentally linked metabolic network model to evaluate the predictive capability of genome-scale metabolic networks for simulating soil microbial communities and seek validation from experimental results. Two approaches with increasing complexity including a variation in carbon sources entering metabolism through different pathways in a liquid flask environment to a spatiotemporal setting is used to predict community composition and dynamics of a four-species community of well-characterized bacterial species. The model reveals a bifurcation in community composition for carbon sources entering metabolism via glycolysis versus directly into central metabolism which is confirmed by experimental results of a synthetic community quantified using qPCR. Finally, dynamic shifts in community composition and spatial patterns are observed when growing the community in a pore network resembling soil. In addition, Appendix 1 discusses the potential for using spatially-linked microbial consortia including mathematical approaches as a new paradigm for biotechnological engineering.
7
1.4 Emerging spatial patterns in sessile bacterial range expansion
Soil is unsaturated for the majority of the time, during which thin water films limit bacterial dispersal and aqueous nutrient diffusion16. In contrast to traditional laboratory settings, microbial life in unsaturated soil is limited to surface-attached growth (biofilms) where favorable spatial positioning and related nutrient availability play a dominant role in colony proliferation and potential interspecies trophic interactions. Colony expansion into previously uninhabited space, a process termed range expansion62, is driven by pioneering cells at the colony periphery which expand outwards from their original position due to a combination of shoving and cell growth63. These pioneering cells and their progeny create a pattern of genetic memory reaching from the original founder cells to the colony periphery64. Emerging sectors of genetically identical cells can be visualized using fluorescently tagged isogenic mutants which take the form of broad sectors expanding perpendicularly to the colony expansion front when competing for the same nutrients64,65. In natural communities however, bacterial cells frequently engage in cross-feeding interactions66,67, thereby shaping their immediate surroundings – a process generally termed niche construction68–70. Cross-feeding interactions impose an additional constraint on the emerging colony pattern by requiring spatial proximity of the interacting partners, which results in a higher degree of intermixing of the two species compared to the competing case outlined above, favoring shorter diffusion lengths71,72. These patterns mediate many aspects of community functioning, and perturbations in the spatial patterns may result in community breakdown and loss of ecological function71,73. In addition to modulating emergent community patterns, range expansion also shapes population genetics of sessile assemblages through genetic drift65. Due to genetic demixing during range expansion, the resulting genetic variation at the growing colony periphery stands in stark contrast to the initial community with consequences for the fate of individual mutations74 and altering the standing variation for natural selection to act upon75. In Chapter 5 we strategically investigate the influence of trophic interactions on the emergent spatial self-organization of a synthetic microbial community using an individual-based mathematical framework including a simple shoving algorithm and nutrient diffusion. We find a unified mechanism giving rise to multiple well-documented patterns rooted in spatially localized growth-rate differences due to the underlying heterogeneous nutrient landscape. In addition, we discuss the results concerning the proliferation of individual lucky cells and genetic consequences of a structured habitat for evolutionary mechanisms.
8
1.5 Generation time distribution in soil
As previously described, bacterial cells are distributed heterogeneously within soil, covering less than 1% of the available soil surface22 and resulting in dense assemblages with disproportional activity (hotspots)3. The remaining 99% of soil surfaces are sparsely inhabited with virtually no bacterial activity (cold spots). Despite being in close spatial proximity of a few microns, these two habitats (hotspots and cold spots) differ fundamentally concerning environmental factors shaping bacterial life – spearheaded by the availability of nutrients. Rapid cell proliferation is enabled in hotspots due to localized availability of carbon and nutrients, whereas low nutrient fluxes render growth in cold spots nigh impossible76. This is reflected in bacterial growth rates measured in situ77, where only growth rates in microbial hotspots (e.g. the rhizosphere78) resemble the copiotrophic growth frequently observed in laboratory settings. Multiple techniques have been used to estimate bacterial growth rates in soil and the subsurface such as viable counts79, isotope-labeled amino acids77, mutation rates80 or more recently via quantitative stable isotope probing using [18O]water with the ability to distinguish between different taxons81. A general limitation of the methods described above is their inability to capture the distribution of generation times and only provide a mean estimate of growth rates in a given volume of soil. The distribution of generation times is primarily interesting in the context of soil viewed as a genetic reservoir, where cells originating from the same ancestor but proliferating at vastly different rates remain in close proximity and result in a genetic archive through time.
Chapter 6 of this dissertation aims to provide a perspective on generation times of soil bacteria supported by simulations employing the mathematical model described in Chapter 3 to probe the effects of nutrient and hydration conditions strategically. The model predicts a wide range of generation times following a power law even within the same species depending on localization within the pore network and related nutrient fluxes. The generation time distribution is shaped by hydration conditions, which dictate the nutrient landscape and redox conditions. Although this analysis is far from painting the whole picture, it provides evidence for how simple mechanisms give rise to wide bacterial age distributions and hints at potential consequences for the genetic memory of soils.
9
1.6 References
1. Curtis, T. P., Sloan, W. T. & Scannell, J. W. Estimating prokaryotic diversity and its limits. Proc. Natl. Acad.
Sci. U. S. A. 99, 10494–10499 (2002).
2. Whitman, W. B., Coleman, D. C. & Wiebe, W. J. Prokaryotes: The unseen majority. Proc. Natl. Acad. Sci.
95, 6578–6583 (2002).
3. Kuzyakov, Y. & Blagodatskaya, E. Microbial hotspots and hot moments in soil: Concept & review. Soil Biol.
Biochem. 83, 184–199 (2015).
4. Jones, D. L., Hodge, A. & Kuzyakov, Y. Plant and mycorrhizal regulation of rhizodeposition. New Phytol.
163, 459–480 (2004).
5. Hinsinger, P., Bengough, A. G., Vetterlein, D. & Young, I. M. Rhizosphere: Biophysics, biogeochemistry and ecological relevance. Plant Soil 321, 117–152 (2009).
6. Kögel-Knabner, I. The macromolecular organic composition of Plant and microbial residues as inputs to soil organic matter. Soil Biol. Biochem. 34, 139–162 (2002).
7. Tiunov, A. V. & Scheu, S. Microbial respiration, biomass, biovolume and nutrient status in burrow walls of Lumbricus terrestris L. (Lumbricidae). Soil Biol. Biochem. 31, 2039–2048 (1999).
8. Tiunov, A. V. & Scheu, S. Carbon availability controls the growth of detritivores (Lumbricidae) and their effect on nitrogen mineralization. Oecologia 138, 83–90 (2004).
9. Schrader, S., Rogasik, H., Onasch, I. & Jégou, D. Assessment of soil structural differentiation around earthworm burrows by means of X-ray computed tomography and scanning electron microscopy.
Geoderma 137, 378–387 (2007).
10. Six, J., Bossuyt, H., Degryze, S. & Denef, K. A history of research on the link between (micro)aggregates, soil biota, and soil organic matter dynamics. Soil Till. Res. 79, 7–31 (2004).
11. Six, J. & Paustian, K. Aggregate-associated soil organic matter as an ecosystem property and a measurement tool. Soil Biol. Biochem. 68, A4 (2014).
12. Parkin, T. B. Soil microsites as a source of denitrification variability. Soil Sci. Soc. Am. J. 51, 1194–1199 (1987).
13. Conrad, R. Soil microorganisms as controllers of atmospheric trace gases (H2, CO, CH4, OCS, N2O, and NO). Microbiol. Mol. Biol. Rev. 60, 609–640 (1996).
14. Zausig, J., Stepniewski, W. & Horn, R. Oxygen Concentration and Redox Potential Gradients in Unsaturated Model Soil Aggregates. Soil Sci. Soc. Am. J. 57, 908 (1993).
15. Or, D., Smets, B. F., Wraith, J. M., Dechesne, A. & Friedman, S. P. Physical constraints affecting bacterial habitats and activity in unsaturated porous media - a review. Adv. Water Resour. 30, 1505–1527 (2007).
16. Tecon, R. & Or, D. Biophysical processes supporting the diversity of microbial life in soil. FEMS Microbiol.
Rev. 41, 599–623 (2017).
17. Skopp, J., Jawson, M. D. & Doran, J. W. Steady-state aerobic microbial activity as a function of soil water content. Soil Sci. Soc. Am. J. 54, 1619–1625 (1990).
18. Ebrahimi, A. & Or, D. Hydration and diffusion processes shape microbial community organization and function in model soil aggregates. Water Resour. Res. 51, 9804–9827 (2015).
19. Dechesne, A., Wang, G., Gulez, G., Or, D. & Smets, B. F. Hydration-controlled bacterial motility and dispersal on surfaces. Proc. Natl. Acad. Sci. 107, 14369–14372 (2010).
20. Ebrahimi, A. N. & Or, D. Microbial dispersal in unsaturated porous media: Characteristics of motile bacterial cell motions in unsaturated angular pore networks. Water Resour. Res. 50, 7406–7429 (2014).
21. Nunan, N., Wu, K., Young, I. M., Crawford, J. W. & Ritz, K. In situ spatial patterns of soil bacterial populations, mapped at multiple scales, in an arable soil. Microb. Ecol. 44, 296–305 (2002).
22. Young, I. M. & Crawford, J. W. Interactions and self-organization in the soil-microbe complex. Science 304, 1634–1637 (2004).
10
23. Ruamps, L. S., Nunan, N. & Chenu, C. Microbial biogeography at the soil pore scale. Soil Biol. Biochem.
43, 280–286 (2011).
24. Fuhrman, J. A. Microbial community structure and its functional implications. Nature 459, 193–199 (2009).
25. O’Donnell, A. G., Young, I. M., Rushton, S. P., Shirley, M. D. & Crawford, J. W. Visualization, modelling and prediction in soil microbiology. Nat. Rev. Microbiol. 5, 689–699 (2007).
26. Borer, B., Tecon, R. & Or, D. Spatial organization of bacterial populations in response to oxygen and carbon counter-gradients in pore networks. Nat. Commun. 9, (2018).
27. Nunan, N., Ritz, K., Rivers, M., Feeney, D. S. & Young, I. M. Investigating microbial micro-habitat structure using X-ray computed tomography. Geoderma 133, 398–407 (2006).
28. Wang, G. & Or, D. Hydration dynamics promote bacterial coexistence on rough surfaces. ISME J. 7, 395–
404 (2013).
29. Kim, M. & Or, D. Individual-Based Model of Microbial Life on Hydrated Rough Soil Surfaces. PLoS One 11, e0147394 (2016).
30. Tuller, M., Dani, O. & Dudley, L. M. Adsorption and capillary condensation in porous media: Liquid retention and interfacial configurations in angular pores. Water Resour. Res. 35, 1949–1964 (1999).
31. Hellweger, F. L. & Bucci, V. A bunch of tiny individuals — Individual-based modeling for microbes. Ecol.
Modell. 220, 8–22 (2008).
32. Hellweger, F. L., Clegg, R. J., Clark, J. R., Plugge, C. M. & Kreft, J. U. Advancing microbial sciences by individual-based modelling. Nat. Rev. Microbiol. 14, 461–471 (2016).
33. Borer, B., Tecon, R. & Or, D. Spatial organization of bacterial populations in response to oxygen and carbon counter-gradients in pore networks. Nat. Commun. 9, (2018).
34. Wang, G. & Or, D. Trophic interactions induce spatial self-organization of microbial consortia on rough surfaces. Sci. Rep. 4, 6757 (2014).
35. Ebrahimi, A. & Or, D. Microbial community dynamics in soil aggregates shape biogeochemical gas fluxes from soil profiles - upscaling an aggregate biophysical model. Glob. Chang. Biol. 22, 3141–3156 (2016).
36. Kreft, J. U., Booth, G. & Wimpenny, J. W. T. BacSim, a simulator for individual-based modelling of bacterial colony growth. Microbiology 144, 3275–3287 (1998).
37. Kreft, J. U. Biofilms promote altruism. Microbiology 150, 2751–2760 (2004).
38. Lardon, L. A. et al. iDynoMiCS: Next-generation individual-based modelling of biofilms. Environ.
Microbiol. 13, 2416–2434 (2011).
39. Orth, J. D., Thiele, I. & Palsson, B. Ø. What is flux balance analysis? Nat. Biotechnol. 28, 245–248 (2010).
40. Edwards, J. S. & Palsson, B. O. Systems properties of the Haemophilus influenzae Rd metabolic genotype.
J. Biol. Chem. 274, 17410–17416 (1999).
41. Gu, C., Kim, G. B., Kim, W. J., Kim, H. U. & Lee, S. Y. Current status and applications of genome-scale metabolic models. Genome Biology 20, 121 (2019).
42. Büchel, F. et al. Path2Models: Large-scale generation of computational models from biochemical pathway maps. BMC Syst. Biol. 7, 116 (2013).
43. Magnúsdóttir, S. et al. Generation of genome-scale metabolic reconstructions for 773 members of the human gut microbiota. Nat. Biotechnol. 35, 81–89 (2017).
44. Machado, D., Andrejev, S., Tramontano, M. & Patil, K. R. Fast automated reconstruction of genome-scale metabolic models for microbial species and communities. Nucleic Acids Res. 46, 7542–7553 (2018).
45. Norsigian, C. J. et al. BiGG Models 2020: multi-strain genome-scale models and expansion across the phylogenetic tree. Nucleic Acids Res. 48 (D1), D402–D406 (2020).
46. Henson, M. A. Genome-scale modelling of microbial metabolism with temporal and spatial resolution.
Biochem. Soc. Trans. 43, 1164–1171 (2015).
11
47. Harcombe, W. R. et al. Metabolic resource allocation in individual microbes determines ecosystem interactions and spatial dynamics. Cell Rep. 7, 1104–1115 (2014).
48. Cole, J. A., Kohler, L., Hedhli, J. & Luthey-Schulten, Z. Spatially-resolved metabolic cooperativity within dense bacterial colonies. BMC Syst. Biol. 9, 1–17 (2015).
49. Bauer, E., Zimmermann, J., Baldini, F., Thiele, I. & Kaleta, C. BacArena: Individual-based metabolic modeling of heterogeneous microbes in complex communities. PLoS Comput. Biol. 13, (2017).
50. Borer, B., Ataman, M., Hatzimanikatis, V. & Or, D. Modeling metabolic networks of individual bacterial agents in heterogeneous and dynamic soil habitats (IndiMeSH). PLOS Comput. Biol. 15, e1007127 (2019).
51. Handelsman, J. Metagenomics: Application of Genomics to Uncultured Microorganisms. Microbiol. Mol.
Biol. Rev. 68, 669–685 (2004).
52. Biggs, M. B., Medlock, G. L., Kolling, G. L. & Papin, J. A. Metabolic network modeling of microbial communities. Wiley Interdiscip. Rev. Syst. Biol. Med. 7, 317–334 (2015).
53. Kreft, J. U. et al. From genes to ecosystems in microbiology: Modeling approaches and the importance of individuality. Front. Microbiol. 8, 2299 (2017).
54. Freilich, S. et al. Competitive and cooperative metabolic interactions in bacterial communities. Nat.
Commun. 2, 587–589 (2011).
55. Chiu, H. C., Levy, R. & Borenstein, E. Emergent Biosynthetic Capacity in Simple Microbial Communities.
PLoS Comput. Biol. 10, (2014).
56. Pacheco, A. R., Moel, M. & Segrè, D. Costless metabolic secretions as drivers of interspecies interactions in microbial ecosystems. Nat. Commun. 10, (2019).
57. Zhuang, K. et al. Genome-scale dynamic modeling of the competition between Rhodoferax and Geobacter in anoxic subsurface environments. ISME J. 5, 305–316 (2011).
58. Bordbar, A., Lewis, N. E., Schellenberger, J., Palsson, B. & Jamshidi, N. Insight into human alveolar macrophage and M. tuberculosis interactions via metabolic reconstructions. Mol. Syst. Biol. 6, 422 (2010).
59. Brune, K. D. & Bayer, T. S. Engineering microbial consortia to enhance biomining and bioremediation.
Front. Microbiol. 5, 203 (2012).
60. Kuzyakov, Y. & Domanski, G. Carbon input by plants into the soil. Review. J. Plant Nutr. Soil Sci. 163, 421–
431 (2000).
61. Brown, G. G. G., Barois, I. & Lavelle, P. Regulation of soil organic matter dynamics and microbial activity in the drilosphere and the role of interactionswith other edaphic functional domains. Eur. J. Soil Biol. 36, 177–198 (2000).
62. Excoffier, L., Foll, M. & Petit, R. J. Genetic Consequences of Range Expansions. Annu. Rev. Ecol. Evol. Syst.
40, 481–501 (2009).
63. Flemming, H. C. et al. Biofilms: An emergent form of bacterial life. Nat. Rev. Microbiol. 14, 563–575 (2016).
64. Weinstein, B. T., Lavrentovich, M. O., Möbius, W., Murray, A. W. & Nelson, D. R. Genetic drift and selection in many-allele range expansions. PLoS Comput. Biol. 13, e1005866 (2017).
65. Hallatschek, O., Hersen, P., Ramanathan, S. & Nelson, D. R. Genetic drift at expanding frontiers promotes gene segregation. Proc. Natl. Acad. Sci. 104, 19926–19930 (2007).
66. D’Souza, G. et al. Ecology and evolution of metabolic cross-feeding interactions in bacteria. Nat. Prod.
Rep. 35, 455–488 (2018).
67. Smith, N. W., Shorten, P. R., Altermann, E., Roy, N. C. & McNabb, W. C. The Classification and Evolution of Bacterial Cross-Feeding. Front. Ecol. Evol. 7, 153 (2019).
68. Buser, C. C., Newcomb, R. D., Gaskett, A. C. & Goddard, M. R. Niche construction initiates the evolution of mutualistic interactions. Ecol. Lett. 17, 1257–1264 (2014).
69. San Roman, M. & Wagner, A. An enormous potential for niche construction through bacterial cross-
12
feeding in a homogeneous environment. PLoS Comput. Biol. 14, e1006340 (2018).
70. Estrela, S. et al. Environmentally Mediated Social Dilemmas. Trends Ecol. Evol. 34, 6–18 (2019).
71. Tecon, R. & Or, D. Cooperation in carbon source degradation shapes spatial self-organization of microbial consortia on hydrated surfaces. Sci. Rep. 7, 43726 (2017).
72. Goldschmidt, F., Regoes, R. R. & Johnson, D. R. Metabolite toxicity slows local diversity loss during expansion of a microbial cross-feeding community. ISME J. 12, 136–144 (2018).
73. Haagensen, J. A. J., Hansen, S. K., Christensen, B. B., Pamp, S. J. & Molin, S. Development of spatial distribution patterns by biofilm cells. Appl. Environ. Microbiol. 81, 6120–6128 (2015).
74. Travis, J. M. J. et al. Deleterious mutations can surf to high densities on the wave front of an expanding population. Mol. Biol. Evol. 24, 2334–2343 (2007).
75. Gralka, M. et al. Allele surfing promotes microbial adaptation from standing variation. Ecology Letters 19, 889–898 (2016).
76. Kieft, T. L. Size Matters: Dwarf Cells in Soil and Subsurface Terrestrial Environments in Nonculturable Microorganisms in the Environment 19–46 (2000).
77. Rousk, J. & Bååth, E. Growth of saprotrophic fungi and bacteria in soil. FEMS Microbiol. Ecol. 78, 17–30 (2011).
78. Bennett, R. A. & Lynch, J. M. Bacterial Growth and Development in the Rhizosphere of Gnotobiotic Cereal Plants. Microbiology 125, 95–102 (1981).
79. Kieft, T. L. & Phelps, T. J. Life in the slow lane: Activities of microorganisms in the subsurface. Chapter 9 in The Microbiology of the Terrestrial Deep Subsurface (2018).
80. Gibson, B., Wilson, D. J., Feil, E. & Eyre-Walker, A. The distribution of bacterial doubling times in the wild.
Proc. R. Soc. B Biol. Sci. 285, (2018).
81. Purcell, A. M. et al. Quantitative Stable Isotope Probing with H218O to Measure Taxon-Specific Microbial Growth. Methods Soil Anal. 4, (2019).
13
Chapter 2
Spatial organization of bacterial populations in response to oxygen and
carbon counter-gradients in pore networks
14
2.1 Abstract
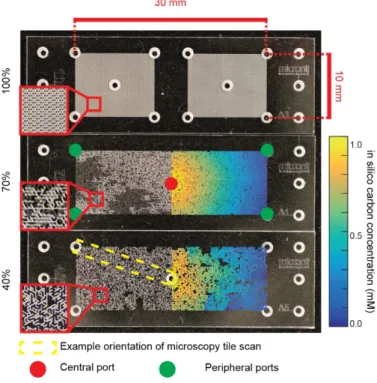
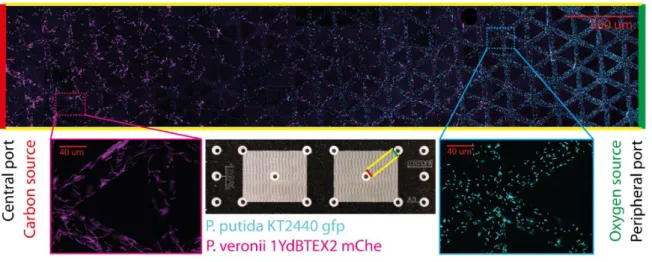
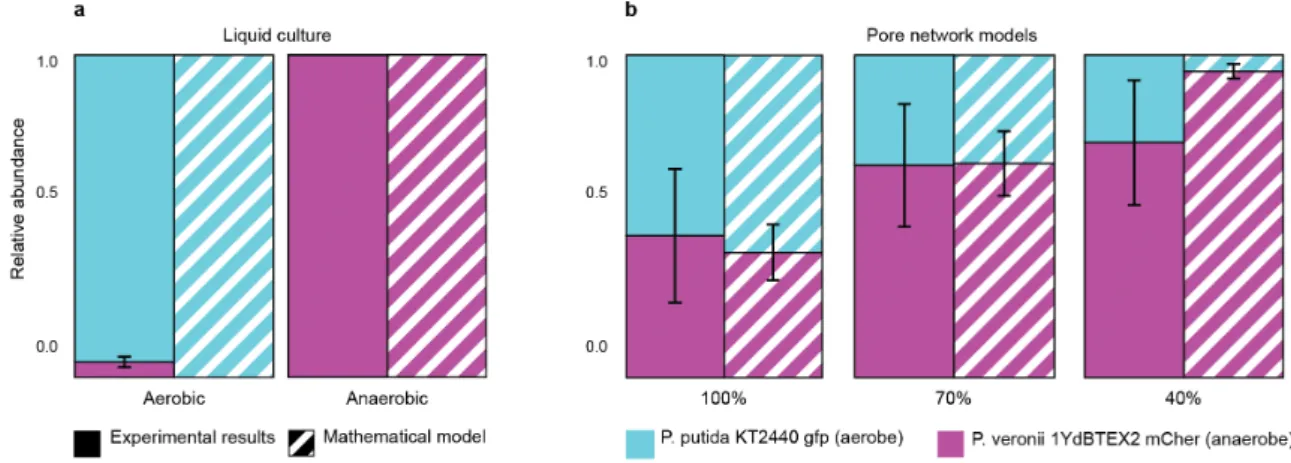
Microbial activity in soil is spatially heterogeneous, often forming spatial hotspots that contribute disproportionally to biogeochemical processes. Evidence suggests that bacterial spatial organization contributes to the persistence of anoxic hotspots even in unsaturated soils. Such processes are difficult to observe in situ at the microscale, hence mechanisms and time scales relevant for bacterial spatial organization remain largely qualitative. Here we develop an experimental platform based on glass- etched micrometric pore networks that mimics resource gradients postulated in soil aggregates to observe spatial organization of fluorescently tagged aerobic and facultative anaerobic bacteria. Two initially intermixed bacterial species, Pseudomonas putida and Pseudomonas veronii, segregate into preferential regions promoted by opposing gradients of carbon and oxygen (such persistent coexistence is not possible in well-mixed cultures). The study provides quantitative visualization and modeling of bacterial spatial organization within aggregate-like hotspots, a key step towards developing a mechanistic representation of bacterial community organization in soil pores.
Borer, B., Tecon, R. & Or, D. Spatial organization of bacterial populations in response to oxygen and carbon counter-gradients in pore networks. Nature Communications (2018).
15
2.2 Introduction
Soil is a living and evolving ecosystem, hosting the greatest abundance and diversity of microbial life in the biosphere and serving numerous regulatory and provisional functions essential for life1,2. Microbes are at the core of soil ecological functioning; they drive key biogeochemical cycles of carbon and nitrogen, and regulate other fluxes important for plant function3. The dynamics, composition and distribution of soil microbes are shaped by heterogeneous water and resource distribution, and by their ability to rapidly adapt to dynamic changes in local conditions4. These intricate local interactions give rise to dense biological hotspots5 that often coincide with nutrient-rich zones such as the rhizosphere6,7, detritusphere8, biopores9–11 and soil aggregates5,12–14. Microbial activity in hotspots accounts for a large portion of soil biogeochemical fluxes; for example, the production of nitrous oxide within a soil core is dominated by denitrification hotspots in which 1% of the soil volume may account for up to 90% of the denitrification activity15. The localized and intense microbial activity within small soil volumes give rise to the formation of anoxic regions. Diffusing oxygen is consumed directly at the periphery of such hotspots16,17, which in turn promotes segregation of aerobes and anaerobes within the local soil microbial community18. This spatial organization of bacterial cells combined with high density of microbial activity promotes and sustains anoxic zones even in aerated soils19 for example in drained rice paddy soils20 or within the rhizosphere17. Soil aggregates have received considerable attention in the past14, concerning their internal chemical conditions16,21, and the distributions of carbon22,23 and bacterial communities24–26.
The formation of microbial community spatial patterns at relevant (micro)scale has been studied over the past decades, with several recent mechanistic models27–30 and experiments31–35 highlighting the importance of spatial organization processes and functional implications26. Here, spatial organization represents the emergence of microbial community patterns that stem from local interactions36. These recent studies have hinted at key factors that promote spatial organization of microbial consortia, including (but not limited to) nutrient spatial gradients, trophic dependencies and chemotactic cellular motility37. Evidence suggests that microbial communities within soil aggregates spatially organize in response to diffusion in 3D pore networks with biologically mediated oxygen gradients16,21. The potential role of persistent and opposing gradients (hereafter counter-gradients) of oxygen and carbon on microbial function has been demonstrated experimentally by glucose perfusion (aimed to disrupt established carbon gradients) with marked changes in microbial activity for different aggregate sizes24. These observations are supported by studies of microbial community composition that show organized species distribution at the pore scale25,38,39. Despite the importance of such microscale spatial organization processes, evidence of bacterial organization in soil pores remains elusive due to the opaque nature of soil particles and difficulties of in situ visualization. Stable spatial patterns of microbes can emerge from an initially homogeneously distributed community as the result of