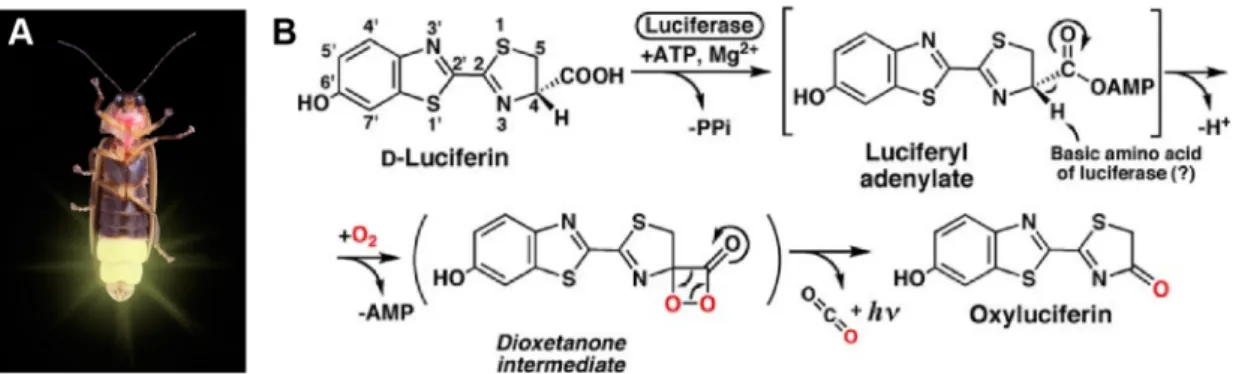
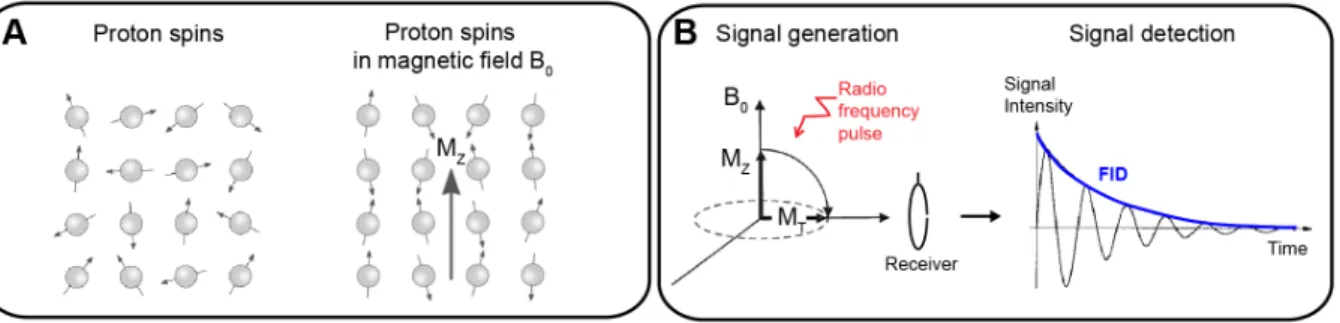
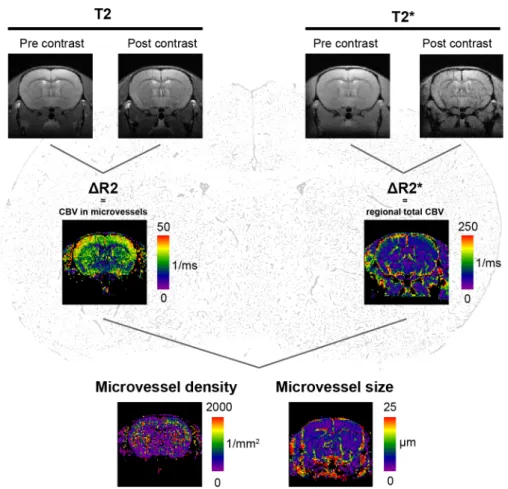
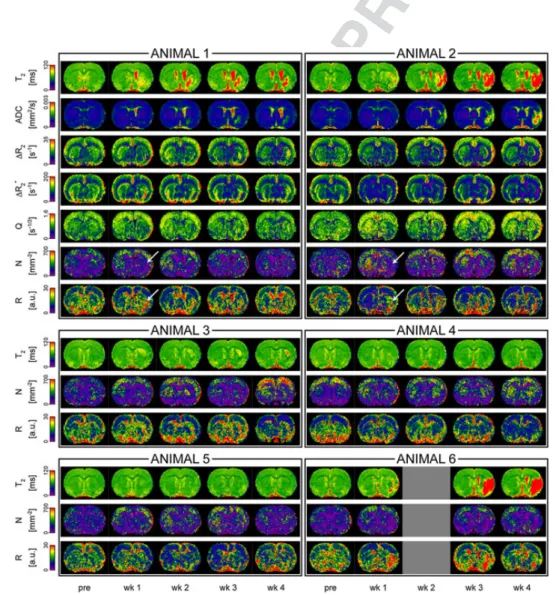
WATCHING THE HEALING BRAIN: MULTIMODAL AND NON-INVASIVE IMAGING OF REGENERATIVE PROCESSES AFTER EXPERIMENTAL CEREBRAL ISCHEMIA
Volltext
Abbildung
ÄHNLICHE DOKUMENTE
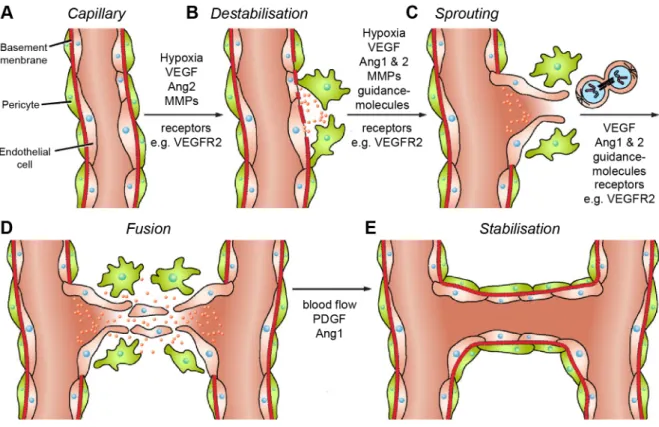
Fruttiger konnte mit Hilfe des Endothelzellmarkers VEGFR1 (vascular endothelial growth factor receptor 1) und des Endothelzell- und Angioblastenmarkers VEGFR2 nachweisen,
Earlier studies conducted in our group showed TrkB was upregulated in spinal cord of oligodendrocyte specific Fgfr1 knockout mice (Rajendran, 2014).. However, in
Im Vergleich mit den verschiedenen Child-Pugh-Stadien zeigten sich nur für VEGF bei Patienten mit einer Zirrhose Child B oder C höhere Mediane als bei gesunden Probanden
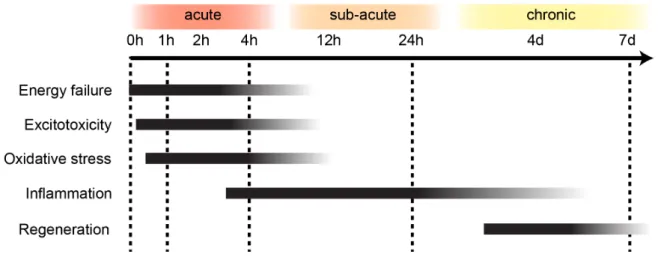
When tissue suffers impaired oxygen supply eventually resulting in cell death, and after focal cerebral ischemia in most cases through necrosis, there is activation of
Evans blue (EB, 50 mg/kg in saline, Sigma–Aldrich, Hamburg, Germany) was injected intravenously in MCAo mice either immediately after or 4, 8, 12 or 16 hours after reperfusion (n =
We have previously shown that total knockout of fibroblast growth factor-2 (FGF-2) results in prolonged survival and improved motor performance in superoxide dismutase 1 (SOD1 G93A
Neben VEGF gilt auch FGF (fibroblast growth factor) als einer der Haupt-Wachstumsfaktoren der Angiogenese in der Plazenta, denn diese beiden Proteinfamilien sind möglicherweise
Diese Annahme wird unterstützt durch Experimente, in denen beispielsweise die Steigerung der Expression von VEGF auf m-RNA-Ebene unter erniedrigtem