DISS. ETH NO. 26665
THE BIOPHYSICAL BASIS OF LATERAL COMPARTMENTALIZATION OF THE YEAST ENDOPLASMIC RETICULUM MEMBRANE
A thesis submitted to attain the degree of
DOCTOR OF SCIENCES of ETH ZURICH (Dr. sc. ETH Zurich)
presented by
ANDRZEJ SLIWA-GONZALEZ MSc, University of Warwick
born on 05.02.1990 citizen of Poland
accepted on the recommendation of Prof. Dr. Yves Barral
Prof. Dr. Karsten Weis Dr. Snezhana Oliferenko
Dr. Bruno Antonny
2020
“The highest forms of understanding we can achieve are laughter and human compassion”
-Richard Feynman
Parts of this thesis have been published in :
• Megyeri M, Prasad R, Volpert G, Sliwa-Gonzalez A, Haribowo AG, Aguilera-Romero A, Riezman H, Barral Y, Futerman AH, Schuldiner M., Yeast ceramide synthases, Lag1 and Lac1, have distinct substrate specificity. Journal of Cell Science, 2019
• Prasad, R., Sliwa-Gonzalez A, Barral, Y. Mapping bilayer thickness in the ER-membrane, Science Advances, 2020
Contributions:
All experiments in this thesis were performed and analyzed by Andrzej Sliwa-Gonzalez, with the exception of data shown in Figure 27B, which were images were produced by Sofia Bachman, Figures 29C, 29D, 34, 40 and 42, which were generated and analyzed by Rupali Prasad ad Figure 46 which were generated and analyzed by Alex Pluess.
Table of Contents
Summary ... 3
Sommario ... 4
Introduction ... 6
The wonderful world of membranes ... 6
The composition of membranes ... 6
Lipids ... 8
Structure of Glycerophospholipids ... 8
Sphingolipids and ceramides ... 12
Biosynthesis of sphingolipids in yeast ... 13
Sterols ... 16
Membrane organization ... 19
Membrane organization and the fluid mosaic model ... 19
The role of sterols in lipid domain formation. ... 23
The roles of sphingolipids and membrane thickness in domain formation. ... 27
How are proteins targeted to lipid domains? ... 33
Budding yeast as a model system to study lipid function and membrane organization ... 43
Diffusion barriers and the lateral compartmentalization of yeast membranes ... 46
The Cortex ... 46
The Endoplasmic Reticulum ... 48
The outer nuclear membrane (ONM) ... 51
Are lipid domains compartmentalizing the yeast ER ... 53
Aim of this work ... 57
Results ... 58
Nsg1-dependent compartmentalization of the outer nuclear membrane (ONM) ... 58
Nsg1 is enriched at the budneck of anaphase nuclei. ... 58
Episomes are more symmetrically partitioned during cell division ... 67
Depletion of membrane proteins at the budneck of anaphase cells is Nsg1-dependent. ... 71
Nsg1 laterally compartmentalizes the ONM during anaphase ... 73
Budneck-independent localization of Nsg1 to the nuclear cleavage plane ... 76
ONM diffusion barrier establishment is Nsg1-dependent, but not budneck dependent. ... 79
Nsg1 interacts with VLCFA synthase components ... 82
VLCFA elongases compartmentalize the yeast nucleus. ... 85
Ceramide synthases differentially compartmentalize the yeast nucleus and cER. ... 87
Development of a membrane thickness sensor ... 89
Proteins with a longer TMD are more present at sites of barrier formation in the cER. ... 90
Construction of Fluorescent ER-membrane reporters of varying TMD lengths ... 93
TMD length-dependent behavior of WALPs in dividing cells ... 94
Development of a membrane thickness sensor- a ratiometric approach ... 97
Long WALPs form foci juxtaposed to the Trans Golgi Network (R. Prasad) ... 105
Ceramide-length dependent compartmentalization of the yeast ER. ... 106
A global reduction of ceramide chain length causes shorter WALPs to associate with the TGN. ... 110
Barrier mutants fail to accumulate ceramide at the budneck ... 113
Discussion ... 115
Is Nsg1 a marker for the ONM barrier? ... 115
Why do ceramide synthases differentially affect the cER and ONM barriers? ... 120
Can we gain any more insights into the nature of the cER diffusion barrier? ... 121
How are the diffusion barriers positioned? ... 125
Materials and Methods ... 129
Strains, Plasmids and Growth conditions. ... 129
Widefield microscopy ... 129
FLIP measurements ... 129
Ratiometric processing of images ... 130
Calculating relative budneck ratio ... 133
Plasmid Propagation Assays ... 133
Protein Complementation Assay ... 133
Strain List ... 134
Appendix 1 ... 139
Acknowledgements ... 142
Bibliography ... 143
Summary
Budding yeast (Saccharomyces cerevisiae) divides asymmetrically, producing a smaller daughter cell with its own cellular identity. During this process, budding yeast laterally compartmentalizes its endomembrane system comprising of the cortical endoplasmic reticulum (cER) and the outer nuclear membrane (ONM) through the action of diffusion barriers at the budneck of dividing cells. Diffusion barriers hinder the exchange of membrane-bound structures between the continuous membrane system shared between mother and bud, ensuring the faithful retention of membrane-bound identity factors to each cell. However, the mode of regulation, as well as the composition of these barriers is currently unknown. The establishment of the ONM and cER diffusion barriers is mediated by various polarity-associated factors, such as the septins and the polarisome component Bud6, and have also recently been shown to also depend on sphingolipids. Furthermore, many ER membrane proteins and certain lipophilic dyes have been shown to be excluded from these membranes at the budneck. This suggests that diffusion barriers might comprise of a specialized lipid domain whose physical properties would hinder the exchange of membrane proteins between mother and bud.
Here we show, that the ergosterol-regulatory enzyme, Nsg1, is specifically involved in the establishment of the ONM diffusion barrier during anaphase. Nsg1 is enriched at the region where the barrier forms in the ONM of anaphase cells and cells lacking this protein have a weakened diffusion barrier. We also show that this enrichment, and the lateral compartmentalization of the ONM associated with it, are budneck-independent. Furthermore, by studying the interaction partners of Nsg1, we were able to show that the ceramide synthases Lac1 and Lag1 differentially affect the establishment of both diffusion barriers.
The involvement of ceramide synthases in barrier establishment led us to develop a reporter system to study the organization of the ER membrane since we postulated that local variations in membrane thickness may be the basis of barrier function. Through the use of genetically-encoded fluorescent membrane peptides, we were able to elucidate the biophysical nature of the cER diffusion barrier and show that membrane thickness is indeed one of the parameters hindering the diffusion of membrane proteins between mother and bud. We also show that this local thickening of the ER membrane is ceramide-dependent and were able to modulate the selectivity of the barrier by a global reduction in the lengths of ceramides the cell produces. Finally, we show that the barrier phenotypes displayed by previously characterized barrier mutants is due to a defect in the establishment of a thicker membrane domain at the budneck.
Based on these results, we propose that variations in membrane thickness are a key parameter of membrane organization in live cells and the local thickening of the ER membrane is a mechanism for restricting the exchange of membrane proteins between the mother cell and bud. Furthermore, these results also show that the organization of the ER is more complex than previously thought.
Sommario
Il lievito di birra (Saccharomyces cerevisiae) si divide asimmetricamente producendo una cellula figlia più piccola con una identità cellulare propria. Durante questo processo, il lievito di birra compartimentalizza letteralmente il suo sistema di endomembrane, composto dal reticolo endoplasmatico corticale (cER) e dalla membrana nucleare esterna (ONM), grazie all’azione di una barriera di diffusione presente al collo del germoglio durante la divisione cellulare. Le barriere di diffusione ostacolano lo scambio di strutture attaccate alla membrana
tra la cellula madre e quella figlia, così facendo assicurano la ritenzione di fattori di identità nel corrispondente compartimento. Ciononostante, il modo in cui le barriere di diffusione vengono regolate e di cosa sono composte rimane sconosciuto. La messa in atto della ONM e del cER è mediata da vari fattori di polarità, come le septine ed il componente Bud6 del polarisoma e gli sfingolipidi, dimostrato di recente. Inoltre, si è mostrato che molte proteine di membrana dell’ER e le tinture lipofili vengono escluse dalle membrane presenti al collo di germoglio. Tutto ciò suggerisce che le barriere di diffusione potrebbero essere composte da un dominio di lipidi specializzato che grazie alle sue proprietà fisiche impedisce lo scambio di proteine di membrana tra cellula madre e cellula figlia.
Qui mostriamo che Nsg1, un enzima regolatore dell’ergosterolo, è coinvolto in modo specifico nella messa in atto delle barriere di diffusione della ONM durante l’anafase. Nsg1 si accumula nella regione dove la barriera di diffusione si forma nella ONM di cellule in anafase, peraltro cellule in cui Nsg1 è rimossa posseggono una barriera di diffusione molto più debole.
Mostriamo anche che questo accumulo e la sua associata compartimentalizzazione laterale della ONM sono indipendenti dal collo di germoglio. Inoltre, studiando i partner di interazione di Nsg1, le sintasi Lac1 e Lag1 influiscono, in modo differente, anch’esse la messa in atto di entrambe le barriere di diffusione.
Il coinvolgimento delle sintasi dei ceramidi nella messa in atto della barriera ci ha condotto a dover sviluppare un sistema reporter per studiare l’organizzazione delle membrane del ER, partendo dal postulato che la variazione locale dello spessore della membrana possa stare alla base per poter funzionare da barriera. Tramite l’uso di peptidi di membrana fluorescenti geneticamente codificati siamo stati in grado di elucidare la natura biofisica della membrana di diffusione del cER e di dimostrare che lo spessore è di fatto uno dei parametri che impedisce la diffusione delle proteine di membrana tra la cellula madre e la cellula figlia. Mostriamo anche che l’inspessimento locale della membrana del ER è ceramide dipendente e che possiamo modulare la selettività della barriera di diffusione grazie ad una riduzione globale della lunghezza dei ceramidi, che la cellula produce. Per finire dimostriamo che i vari fenotipi concernenti la barriera di diffusione sono causati dalla messa in atto di un dominio di membrana più spesso al collo di germoglio.
Partendo da questi risultati proponiamo che le variazioni di spessore della membrana sono il parametro chiave per l’organizzazione delle membrane delle cellule viventi e che
l’inspessimento locale della membrana del ER è un meccanismo per impedire lo scambio di proteine di membrana la cellula madre e germoglio. Inoltre, questi risultati dimostrano anche che l’organizzazione del ER è più complessa di quello che si pensava in precedenza.
Introduction
The wonderful world of membranes
There is no life without membranes. At their core, cellular membranes are nothing more than a mixture of lipids forming a matrix (the lipid bilayer) where other molecules such as proteins and carbohydrates reside. Historically, the main role of membranes has been thought to spatially separate the various biochemical processes happening in the cell from the environment (in the case of the plasma membrane) or to spatially restrict these processes within a cell (in the case of organelles). In other words, if it wasn’t for this simple ‘barrier’ function, the other building blocks of life such as nucleic acids and proteins would be spatially disorganized and could therefore not function properly and bring about ‘life’ as we know it. However, work done on these structures over the past couple of decades has drastically changed our view of membranes from simple barriers shielding the cell from various environments to other non- canonical, yet equally important functions ranging from energy storage and signaling to other less-obvious phenomena such as the organization of various complexes at the membrane (Sezgin, Levental, Mayor, & Eggeling, 2017). In fact, many of these newly described functions depend on the ability of these membranes to self-organize and form different lipid phases, and we are just starting to get a picture of how these processes work and the effects this has on the cell. In fact, it seems as if cellular membranes are involved in every biological known process, and we often find ourselves reexamining previous observations in light of what we presently know about these important cellular structures.
The composition of membranes
All biological membranes are composed of a glycerophospholipid (GPL) bilayer, where proteins and sugars are associated in various ways. Due to the fact that GPLs are polar lipids and therefore amphipathic (i.e. polar on one side and apolar on the opposite side), they are able to spontaneously form bilayers in aqueous environments since one side of the lipid is hydrophilic (and therefore oriented towards the aqueous side), while the hydrophobic/apolar side is oriented away from the aqueous environment (Figure 1). Because the polar head groups are able to interact with the aqueous side and with each other, while the hydrophobic tails are entropically
driven away from the aqueous interface, the spontaneous formation of membranes can happen since this process is energetically favorable. Furthermore, due to the fact that lipids are not covalently linked to each other, the environment formed by the lipid bilayer can be thought as a two-dimensional environment where lipids and proteins diffuse laterally (i.e. across the bilayer, Figure 2). Therefore, membranes are highly dynamic structures and this remarkable characteristic sets membranes as a special type of two-dimensional fluid whose role is not just limited to forming a barrier between two environments, but also forms a platform where different biochemical processes can occur. Indeed, the very fact that many enzymes cannot properly function (Cournia et al., 2015) without a membrane suggests that the role of these structures go beyond their classical ‘barrier’ function.
Figure 1. The formation of membranes by lipids. The hydrophilic head groups of lipids interact with the aqueous environment while the hydrophobic acyl chains are entropically driven away from this environment leading to the formation of a bilayer.
Within a single cell, the many combinations of lipids and proteins giving rise to the various membranes present in that cell endow that particular membrane with its own biochemical identity suited to its biological function. Therefore, in order to understand the behavior and role of membranes in the context of a cell, it is important to understand how the different membrane building blocks interact with one another and are organized at the topological level and more importantly, how the small structural differences in individual building blocks exert their function at the macromolecular level (i.e. at the level of the membrane).
Figure 2. Lateral Diffusion of lipids in a bilayer. A single lipid (red) is able to laterally diffuse across the bilayer.
Lipids
The field of lipid research has exploded in the past decade, mainly owing to technological advances and over 20,000 biologically relevant lipids have been identified up to date (Fahy et al., 2009). However, the three main classes of lipids relevant to membrane biology are the glycerophospholipids (GPLs), sphingolipids (SLs) and sterols. GPLs and sphingolipids are polar lipids, meaning that they can form bilayers and are therefore the main structural building blocks of membranes.
Structure of Glycerophospholipids
Within the polar lipids, GPLs are the most abundant class of structural lipids (by structural we mean lipids forming the bilayer). GPLs are composed of 2 fatty acid chains linked to a glycerol backbone at positions sn-1 and sn-2 (Figure 3). Various types of fatty acids of varying lengths and saturations are present at these positions, although the sn-1 position is usually saturated/mono- unsaturated while the sn-2 position are usually mono/polyunsaturated (Harayama & Riezman, 2018). In terms of nomenclature of these lipids, GPLs are classified according to the structure of the headgroup. Among these, the most prominent modifications are the addition of choline,
serine, inositol, ethanolamine, glycerol, and some sugars such as glucose (Harayama & Riezman, 2018). Thus, the most prominent structural GPLs found in the cell are phosphatidylinositol (PI), phosphatidylcholine (PC), phosphatidylserine (PS) and phosphatidylethanolamine (PE) (Figure 3).
Additionally, some GPLs such as phosphatidylinositol are further modified by phosphorylation at various positions giving rise to different classes of PIs which are involved in signaling, lipid transport, membrane identity and the anchoring of various proteins to the plasma membrane.
The latter of these functions is due the fact that the functional groups of inositol can also serve as platforms for the covalent linkage to the C-terminus of proteins, as is the case for GPI-anchored proteins (Balla, 2013). Interestingly, while PC is thought to be the most abundant membrane GPL in eukaryotic cells with some estimates ranging from 41-47 % depending on the organelle of interest (van Meer, Voelker, & Feigenson, 2008), the most prominent GPL species in budding yeast are the PIs , constituting ~20 mol% while PC and PE are slightly less abundant (~14 mol%;
(Ejsing et al., 2009)).
It is important to notice that this modular nature of GPLs has a tremendous impact in terms of the number of GPL species which can be synthesized by the cell. This diversity results from the variety of fatty acids which can be linked at the sn-1 and sn-2 positions, which differ in the number of carbons present in the acyl chain (acyl-chain length), the number of double bonds or unsaturation state of these chains as well as the position of this double bond. Furthermore, the addition of a head group to the phosphate moiety of GPLs further expands the GPL repertoire and these head groups can have different effects on membrane properties. For example, some modifications such as the addition to choline or ethanolamine, which are zwitterionic (i.e. they have more than one functional group) modify the physical and chemical properties of these lipids since the presence of two opposite charges renders the head group neutral while other modifications such as serine give a negatively charged headgroup.
Figure 3. Structure of GPLs. The corresponding structural characteristics of GPLs have been color coded (blue;
head group, green and red are the fatty acids associated at positions sn-1 and sn-2 respectively). The headgroups for PE, PC, PS and PI are also depicted in blue.
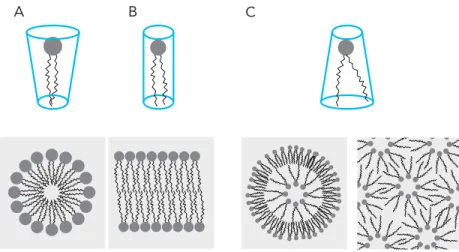
Most importantly, different combinations of these aforementioned parameters such as the size and shape of the headgroup and the length/saturation of its acyl chains give lipids specific shapes which in turn can dictate the supramolecular assemblies it can form in an aqueous environment, a term otherwise known as lipid polymorphisms (Frolov, Shnyrova, & Zimmerberg, 2011). The shapes lipids can assume can be broadly classified into conical (where the “bottom”
hydrophobic part of the lipid is wider than the “top”), inverted conical (the hydrophilic part is wider than the hydrophobic) and cylindrical (roughly equal widths at the “top” and “bottom” part of the lipid). While cylindrical lipids, such as PC, tend to form lamellar phases (a type of lipid polymorphism characteristic for bilayers), other polymorphisms exists such as micellar phases where the thinner hydrophobic chains pack towards the center of the micelle with the headgroups on the outside, in the case of PIs, and cubic lipid phases which are more complex in structure but characterized by a 3D assembly with regular aqueous channels protruding throughout this structure in the case of the inverted conical lipid, PE (Figure 4) (Jouhet, 2013).
What then, is the role of these non-lamellar lipids in membranes and what is the purpose of synthesizing them if they have a detrimental effect on the formation of the bilayer? There are
CH
C [CH]n
CH CH
CH2 C O
O O O PO3
R CH2 CH2
[CH]n`
[CH]n``
CH2
CH CH NH2
CH CH NH3+
NH2 COO-
CH OH
OHOH OH OH Ethanolamine
Serine Inositol
Choline
several theories which can explain this. One explanation lends itself from the field of complex systems theory, which states that single component systems (for example a bilayer made up of only PC) are not robust enough to withstand constant perturbations, such as changes in osmolarity, pH and temperature. Because the main function of membranes is to create a stable boundary between two opposing and more often than not, unstable environments, then having a complex mixture of lipids could buffer some of these perturbations. Furthermore, the presence and insertion of transmembrane proteins as well as various cellular processes such as the fusion of two different membranes poses a significant local energetic constraint on the bilayer. Since in these instances, local variations in membrane curvature and shape take place, the presence of various lipid shapes may help to stabilize the bilayer (Sampaio et al., 2011). Interestingly, it has long been hypothesized that the presence of a complex lipidome allows the cell to generate membranes with different properties which underlie the vast morphological diversity of organelles present in cells. Indeed, evidence from the field of evolutionary biology shows that the emergence of more sophisticated cellular organelles in eukaryotes coincides with the emergence of a more complex lipidomes which include lipid species not present in prokaryotes such as SLs and sterols (Gould, 2018).
Figure 4. Lipid polymorphisms. The three different lipid shapes; conical (A) lipids form micellar phases (bottom part), cylindrical lipids (B) form lamellar phases while inverted conical lipids (C) form more complex inverted phases.
A B C
Sphingolipids and ceramides
The second class of structural polar membrane lipids are the sphingolipids (SLs). In contrast to GPLs whose structural backbone is a diacylglycerol (DAG) moiety, SLs consist of a sphingoid base (otherwise known as a long chain base or LCB), which is the product of condensation of L-serine with palmitoyl-CoA. SLs exhibit the same amphipathic nature as GPLs, yet there are many important differences such as the presence of longer saturated acyl chains, endowing these lipids with unique physicochemical properties. In eukaryotic cells, the most prominent LCB is sphingosine, which is characterized by a C18 aliphatic chain, and hydroxyl groups at positions 1 and 3 and a double bond at position 4. The generation of a variety of other LCBs is possible through the attachment of different fatty acids as well as the addition of hydroxyl groups or double bonds at different positions further expanding the chemical/physical characteristics of these lipids. For example, DHS (dihydrosphingosine) and PHS (phytohydrosphingosine) all contain a palmitoyl fatty acid chain but differ in the hydroxylation state at position 4, and this small difference makes PHS more prone to form membrane domains (Marques et al., 2015). SLs also consist of a N-acyl-linked fatty acid moiety as well as a headgroup at position 1. While the length and saturation state of the N-linked fatty acyl chain may vary, these are usually long and saturated in contrast to GPLs, which usually exhibit shorter acyl chains with varying degrees of saturation (Figure 5). As in GPLs, the large variety of SLs present in nature are due to the modular architecture of SLs and the nomenclature of different SLs is based on their headgroup. Among these, the most common headgroup modifications are hydroxyl groups, phosphocholine, phosphoethanolamine, glucose and galactose giving rise to ceramides, sphingomyelins, ceramide phosphoethanolamides (CerPEs), glucosylceramides (GlcCers) and galactosylceramides (GalCers) respectively. Additionally, oligosaccharide chains can also be added as headgroups giving rise to complex glycosphingolipids (GSLs).
Figure 5. The structure of sphingolipids. A The corresponding structural characteristics of SLs have been color coded (blue; head group, green shows the fatty acid moiety of SLs while red shows the structure of the long chain base). B A comparison between GPLs, complex SLs and ceramide.
While SLs constitute an important portion of cellular membranes, their overall distribution is much more limited than GPLs. Although synthesized in the ER together with many other lipids, SLs are rapidly exported to the plasma membrane where they have important functions including providing mechanical support by enhancing membrane rigidity as well as signaling roles and the formation of membrane domains (Dickson, Nagiec, Skrzypek, et al., 1997;
Dickson, Nagiec, Wells, Nagiec, & Lester, 1997; Jenkins et al., 1997; Lester, Withers, Schultz, &
Dickson, 2013; Megyeri, Riezman, Schuldiner, & Futerman, 2016; Wells, Dickson, & Lester, 1998).
Biosynthesis of sphingolipids in yeast
Similar to GPLs, the synthesis of sphingolipids begins in the ER membrane where L-serine and palmitoyl-CoA are condensed into 3-ketodihydrosphingosine (3-KDS) through the action of the serine palmitoyl transfrase (SPT) complex (Figure 6). This first key step in SL biosynthesis is conserved across all organisms producing SLs (Harrison, Dunn, & Campopiano, 2018). In budding yeast, this complex consists of two subunits, Lcb1 and Lcb2 (Nagiec et al., 1997). This complex is
CH2 NH
CH CH CH CH O
R
OH
C [CH]23
CH CH2 O [CH]14
CH2 Complex SL
16-18 carbons 26 carbons
Ceramide
Acyl chain length Saturation GPL
A
B
also part of a larger complex called the SPOTS complex, which additionally includes the proteins Orm1, Orm2, Tsc3 and Sac1, and is involved in the regulation of LCB levels (Breslow et al., 2010).
Although the molecular details governing LCB regulation by the SPOTS complex are still unclear, it is now known that this complex is involved in a rheostat-like regulation of LCB levels. Orm1 and Orm2 are negative regulators of SL biosynthesis, and their phosphorylation by Ypk1 leads to the disassociation from the SPT complex (Roelants et al., 2017). This in turn releases the SPT from the SPOTS complex resulting in the upregulation of 3-KDS levels (Foti, Audhya, & Emr, 2001;
Hughes et al., 2000).
Figure 6. The yeast SL biosynthesis pathway. Enzymes are color coded red while the various SL precursors are purple.
Once 3-KDS has been synthesized, it is rapidly converted into dihydrosphingosine (DHS) by the 3-ketosphingosine reductase, Tsc10 (T. Beeler et al., 1998). DHS is the first biologically active SL precursor and this is the point where the SL synthesis pathway branches into two distinct branches; namely the DHS pathway, where no hydroxylation of C4 occurs, and the
phytosphingosine (PHS) pathway, where hydroxylation occurs at the C4 carbon by the C4- hydroxylase Sur2 (Haak, Gable, Beeler, & Dunn, 1997). Furthermore, the action of Sur2 is not restricted to hydroxylation of LCBs since the downstream product of DHS, dihydroceramide (DHCer), can also be converted into its hydroxylated version phytoceramide (PHCer) (Megyeri et al., 2016).
Once PHS or DHS have been made, the next important step in this pathway is the formation of the simplest sphingolipid, ceramide, by the ceramide synthases Lag1 and Lac1 (Barz
& Walter, 1999; Jiang, Kirchman, Zagulski, Hunt, & Jazwinski, 1998; Vallee & Riezman, 2005).
Interestingly, the identification of the ceramide synthase Lag1 originally happened in a budding yeast screen for genes whose deletion resulted in an increase of replicative lifespan, and was named as Lifespan Assurance Gene 1 (D'Mello N et al., 1994). Lac1 (Lifespan Assurance gene Cognate 1) on the other hand, although closely related to Lag1 in sequence and function, does not show the same phenotype in terms of aging (D'Mello N et al., 1994). Furthermore, the deletion of Lag1 or Lac1 alone is not lethal, but the double deletion of these genes is lethal depending on the strain background (Alcaide-Gavilán et al., 2018). Nonetheless, the main function of the ceramide synthases is to N-acetylate PHS or DHS giving rise to phytoceramide (PHCer) or dihydroceramide (DHCer). In yeast, the fatty acid elongases Elo2 and Elo3 are responsible for the synthesis of C24 and C26 fatty acids respectively, (Denic & Weissman, 2007;
Guillas et al., 2001) and it is these products which serve as the substrates for the formation of C24 and C26 ceramides by Lag1 and Lac1. Aside from the differences in replicative aging phenotypes, it has recently been shown that Lac1 and Lag1 differ in their substrate specificity;
Lag1 preferentially uses the hydroxylated version of sphingosine (PHS) while Lac1 has preference for DHS (Megyeri et al., 2019). While DHCer and PHCer are the two predominant forms of ceramide found in yeast, both of these species can further be hydroxylated in the C2 position of the acyl chain by Scs7 generating alpha-hydroxyl-DHCer or alpha-hydroxyl-PHCer. A fifth ceramide species has also been described, albeit to a lesser extent, where a hydroxyl group is added at a yet undefined position by Ccc2 yielding alpha-x-hydroxyl-PHCer (T. J. Beeler et al., 1997).
All of the aforementioned steps of the SL biosynthesis pathway happen in the ER membrane. However, once ceramide has been synthesized, the next step in the pathway is to form inositolphosphoceramide (IPC) through the action of the IPC synthase Aur1 and Kei1 (Heidler & Radding, 1995; Nagiec et al., 1997; Sato, Noda, & Yoda, 2009). Although there is some evidence that the majority of Aur1 at steady state levels is in the ER, the conversion of ceramides into IPC derivatives happens in the Golgi apparatus (Megyeri et al., 2016). Therefore, ceramides must be transported to the Golgi prior to IPC synthesis. In mammalian and yeast cells, this process is thought to happen either through vesicular transport from the ER to the Golgi, or through non- vesicular means (Kajiwara et al., 2014). The ceramide transport protein CERT has been found to mediate the non-vesicular transport of ceramide to the Golgi in mammalian cells (Hanada et al., 2003) while in yeast, there is some evidence that the ER-resident protein Nvj2 facilitates the non- vesicular transport of ceramide to the Golgi (Liu, Choudhary, Toulmay, & Prinz, 2017). Once ceramide has reached the Golgi and IPC has been formed, IPC can then be mannosylated by the enzymes Csg1, Csg2 and Csh1 forming the mannosyl-IPCs (MIPCS;(Uemura, Kihara, Inokuchi, &
Igarashi, 2003)). In yeast, this class of sphingolipids is by far the most abundant constituting up to 12 mol% of the lipidome (Ejsing et al., 2009). Furthermore, an additional inositolphosphate group can also be added by Ipt1 resulting in the formation mannosyl di-IPCs (M(IP)2C; (Dickson, Nagiec, Wells, et al., 1997)). Once these different SL species are formed, they are next transported to the PM via the secretory pathway, where they can constitute up to 10% of the PM lipids (Cowart & Obeid, 2007).
Sterols
The third major membrane lipid class are the sterols. These apolar lipids are also known as steroid alcohols and are characterized by a four-ring structure. Due to their apolar nature, these lipids cannot form bilayers but instead are thought to modulate a variety of membrane- associated processes such as vesicle formation, protein sorting, signaling and the formation of lipid domains, most likely through the modulation of membrane fluidity. There are three main classes of sterols present throughout the eukaryotic kingdom (Figure 7). Cholesterol is the major sterol found in animals, while phytosterols and ergosterol are present in plant and fungal cells
respectively (Hu et al., 2017). The fungal sterol, ergosterol, is one of the most studied components of yeast membranes and it has been shown to regulate a variety of biological processes such as mating, endocytosis, membrane fluidity and permeability as well as salt and drug tolerance through the activity of some transporters (Kodedova & Sychrova, 2015). Like SLs, sterols are major components of the PM and are also synthesized in the ER membrane. In budding yeast, where ergosterol is the most abundant lipid (constituting up to 12 mol% of the yeast lipidome; (Ejsing et al., 2009)), the pathways and enzymes responsible for the production and regulation of ergosterol synthesis have been identified. This includes a list of around 30 enzymes, termed the Erg- proteins. Broadly speaking, this pathway can be stratified into 3 main modules, the first being the production of the first major ergosterol intermediate, mevalonate- CoA. The second module involves the production of farnesyl pyrophosphate (FPP) through the action of Erg12, 18, 19 and 20 respectively (Hu et al., 2017). In the last biosynthetic module, consisting of 15 steps, FPP is converted into squalene which is the precursor of lanosterol and finally ergosterol.
Figure 7. The three main classes of sterols. (A) depicts cholesterol while (B) and (C) depict campesterol (a phytosterol) and ergosterol respectively. Structure of cholesterol is color coded in red while any additional modifications in campesterol or ergosterol are color coded with purple (for campesterol) and green (for ergosterol).
A
B C
The first module is conserved throughout all eukaryotes and happens through the condensation of two acetyl-CoA molecules into acetoacetyl-Coa by Erg10 (Hiser, Basson, & Rine, 1994). A third condensation reaction between acetoacetyl-CoA and acetyl-Coa is then catalyzed by Erg13, yielding 3-hydroxy-3-methylglutaryl-CoA (HMG-CoA; (Miziorko, 2011)). The last and perhaps most important step is the formation of mevalonate-CoA by the HGM-CoA reductases, Hmg1 and Hmg2. The importance of this module is due to the fact that this is the main rate- limiting step of ergosterol biosynthesis and therefore responsible for the total amounts of ergosterol in the cell. This last step of mevalonate synthesis is where the regulation of ergosterol levels happen through the action of the yeast INSIG homologues, Nsg1 and Nsg2. In this regulatory module, the ergosterol precursors downstream of mevalonate (such as FPP (farnesylpyrophosphate)) are thought to induce the ERAD-mediated degradation of Hmg’s through the binding of a specific transmembrane motif termed the sterol-sensing domain (SSD).
Whenever sterol levels, and therefore FPP levels are high, HMGs are degraded. However, when sterol levels are low, Nsg1 and Nsg2, which compete for the same sequence motif, are able to inhibit the degradation of HMGs thereby increasing the rate of mevalonate production (Figure 8) (Flury et al., 2005; Theesfeld & Hampton, 2013).
Figure 8. Nsg1/Hmg-mediated regulation of ergosterol levels. Nsg1 is a chaperone for the Hmg’s preventing their degradation. However, when FPP levels are high (meaning that ergosterol levels are also high), they outcompete Nsg1 and promote the degradation of the Hmg’s
HMG-CoA Mevalonate-CoA
FPP Ergosterol
Nsg1 Hmg1 Hmg2 Low Ergosterol = Low FPP
HMG-CoA Mevalonate-CoA
FPP FPP
Ergosterol
Nsg1 Hmg1 Hmg2 High Ergosterol = High FPP
Membrane organization
Membrane organization and the fluid mosaic model
In order to gain a mechanistic understanding of the functions of biological membranes, it is crucial to characterize the complex interactions between the lipids and proteins of membranes, since these are what give membranes their physico-chemical characteristics necessary for their function. In other words, we must understand how the various membrane components are organized at both temporal and spatial scales, and this is what is broadly defined in the field of membrane biology as “membrane organization” (Cheng & Smith, 2019). However, in contrast to the fields of protein and nucleic acid research, this goal is much more difficult since membranes are not organized in the same way as proteins and nucleic acids. Unlike proteins and nucleic acid polymers, membranes are not composed of “poly-lipids” and are therefore unamenable to the same types of sequence/structure analysis. Furthermore, biological membranes are extremely dynamic structures whose composition may temporally change as a function of many parameters such as temperature and pH and cell cycle stage (Klose et al., 2012). Another key difference in the study of membranes is the fact that biological membranes are open systems, meaning that its constituents are in constant flux.
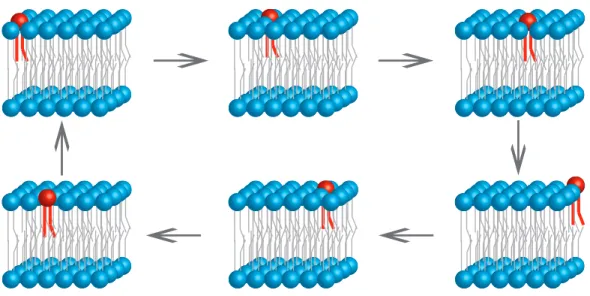
While it may seem that studying membranes is a daunting task (which it is!), our first big step in understanding the organization of membranes came in the form the Fluid Mosaic model, first proposed by Singer and Nicholson in the early 70’s (Singer & Nicolson, 1972). In essence, the FM model stated that biological membranes are organized as a lipid bilayer upon which molecules such as proteins and other lipids are embedded. The term “mosaic”, owes to the fact that lipids, which serve as the main building blocks of bilayers, together with proteins, were thought to form a random heterogenous 2D mixture or “mosaic” (Figure 9). In the original model, the range of motions which molecules in this bilayer can adopt are twofold. Rotational motion or the ability to move around a molecules longitudal axis, and translational, which is the ability of a molecule to freely diffuse along the plane of the bilayer, were thought to be the predominant types of movement permitted by the FM model. Trans bilayer movement, which is the third type of movement which could theoretically occur, describes the movement of molecules from one
leaflet of the bilayer to another leaflet, was not permitted by this model due to the energetic requirements imposed by the hydrophobic core of the bilayer (Figure 10). Therefore, the “fluid”
aspect of membranes is derived from these physical characteristics and is the basis for why we think of membranes as liquid 2D environments. Although there have been many refinements to the FM model since its original inception, the relative success of this model owes to the fact that it is founded purely on already-known physical and chemical characteristics of its constituents, such as the ability of certain lipids to assemble into bilayers. Furthermore, the relative simplicity of this model has made it possible for people studying membrane organization to not only make predictions based on these physical principles, but also to test them experimentally.
Figure 9. A 3-D representation of the FM model. Note the random distribution of lipids (purple, blue and red) and membrane proteins (green).
Figure 10. The three types of motions lipids undergo in a bilayer. (A) Rotational motion, (B) translational diffusion and (C) trans-bilayer movement are three types of lipid motions which can theoretically occur, although only the first two are permitted by the FM model.
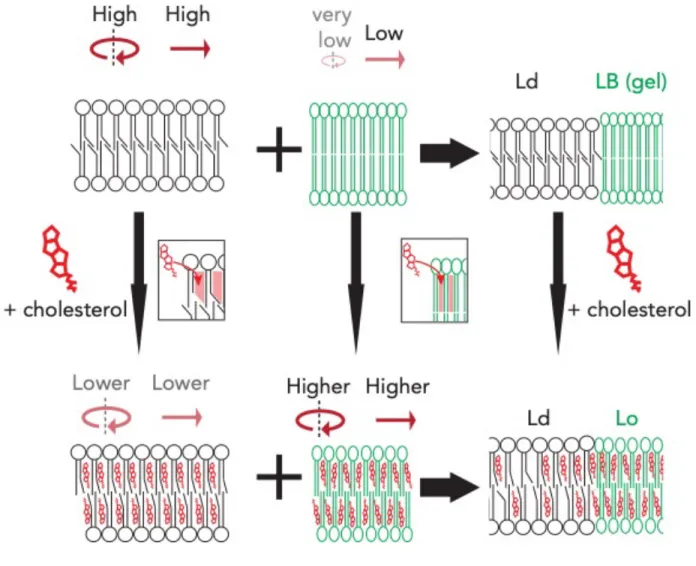
Although the original FM model has undergone many updates since its inception, perhaps the biggest update to the FM model came from the realization that membranes are not as laterally heterogenous as the model originally predicted and this stemmed from both observations of model membrane systems and from early biochemical analysis of red blood cell membranes. In the first of these cases, it was observed that specific mixtures of lipids have the remarkable property to organize into various lamellar phases in vitro. Under specific conditions, ternary lipid mixtures (i.e. containing three lipid species) composed of lipids with saturated acyl chains and high melting temperatures (Tm’s), lipids with unsaturated acyl chains with low Tm’s and cholesterol were found to form two distinct lipid phases: Lo (liquid-ordered) and Ld (liquid- disordered) phases. From a physical perspective, phases are defined as spaces where the physical properties of a given material are the same. In the case of model membranes, Lo phases were characterized by having a high degree of translational diffusion but a low degree of rotational
A
C B
motion, whereas Ld phases are lipid phases where both rotational and translational motions where relatively high (Phillips, Ladbrooke, & Chapman, 1970; Shimshick & McConnell, 1973) (Figure 10). Furthermore, the composition of these two phases was also very different since Ld phases tend to be composed of unsaturated low Tm lipids while the Lo phase was mainly composed of cholesterol and saturated high Tm lipids. Interestingly, these in vitro results were recapitulated in living systems through the biochemical characterization of red blood cell plasma membranes which also showed the differential solubility of membranes according to what detergents are used (Brown & Rose, 1992). Specifically, it was shown that detergent resistant membranes (DRMs) and detergent-soluble membranes could be biochemically differentiated and most importantly, that their compositions where different. The composition of DRMs correlated with the composition of Lo phases in model membranes since these contained a high degree of cholesterol and SLs, which are mostly saturated and have a high Tm, while the detergent-soluble fraction was more compositionally reminiscent of the Ld phase. Furthermore, the protein composition of these two fractions also differed, sparking the idea that this lateral heterogeneity of membranes could have a biological role. These and other observations throughout the 80s led to the formulation of the lipid raft hypothesis by Simons and van Meer (Simons & van Meer, 1988), which proposed that the preferential association of certain lipids such as SLs and cholesterol could drive the formation of lipid domains which would in turn drive the sub compartmentalization of other membrane constituents (Simons & Sampaio, 2011). This idea was revolutionary not because it suggested that there is a higher degree of organization to membranes than what was previously thought, but rather provided means to explaining how membrane processes could be regulated since the formation of these domains is a thermodynamically-driven process dependent on the presence of specific lipids. An important implication of this hypothesis is that the formation of lipid domains could provide membranes the means to not only compartmentalize chemical events across the bilayer, but also along the bilayer (i.e laterally).
While there is still much discussion around the specific aspects of this hypothesis, for example regarding whether lipid-lipid or protein-lipid interactions drive the formation of these structures (Simons & Sampaio, 2011), the notion that there is a high degree of lateral
heterogeneity in cellular membranes has been generally accepted by the field and has served as a useful paradigm for how membrane-associated processes are organized and regulated. It must also be noted that definitive evidence of their existence in living cells has yet to be shown, since these domains are postulated to be anywhere from 10-200 nm in size, which is well below the diffraction limit of most microscopes and any circumstantial evidence of their existence has only been shown for the plasma membrane (Pike, 2006; Varma & Mayor, 1998). Furthermore, while the existence of different lamellar phases has been shown to happen in vitro, usually through the use of biologically-relevant ternary lipid mixtures, it has been a challenge to translate most of these findings to in vivo studies due to the fact that biological membranes are composed of hundreds of fluctuating lipid species (instead of only 3 or 4) and also contain a high degree of membrane proteins which can considerably change the membrane environment. It is also important to note that the raft hypothesis has been mainly used to describe the organization of rich in cholesterol and complex sphingolipids, such as the plasma membrane. However, not all cellular membranes are cholesterol and SL-rich and there is increasing evidence that the formation of lipid domains can also pervade other membranes of eukaryotic cells such as the vacuole in yeast (Toulmay & Prinz, 2013) or in prokaryotes, which don’t synthesize cholesterol and SLs (Bramkamp & Lopez, 2015; Nickels et al., 2017). Regardless of these considerations, the lipid raft model, as in the case of the FM model, is also based on thermodynamic and physical principles explaining how membranes are organized, which in turn has allowed us to characterize the roles of these rafts, as well as other lipid domains, through both experimental and theoretical approaches. While much research has been done on the interplay of membrane lipids and proteins in membrane domain formation, the two main lines of investigation in the field deal with the effect of two particular lipid species on the formation of lipid domains; the SLs and the sterols, as well as the mechanisms of association of proteins with these domains.
The role of sterols in lipid domain formation.
The original raft hypothesis postulated that the preferential association of cholesterol (the main mammalian membrane sterol) with complex SLs such as sphingomyelin was the key
driver of domain formation (Goni, 2014; Pike, 2006; Simons & Sampaio, 2011) .This notion not only stemmed from the fact that these 2 lipid species were identified to be the main components of raft-like membrane fractions (Brown & Rose, 1992; R. Schroeder, London, & Brown, 1994) but also because perturbing the biosynthesis and regulation of these lipids caused a disruption in the organization of raft-associated proteins (R. J. Schroeder, Ahmed, Zhu, London, & Brown, 1998).
Sterols are major modulators of several important bilayer physical parameters such as order, fluidity and thickness (Kaiser et al., 2011), and understanding the mechanistic details regarding how sterols interact with other lipid is crucial in understanding how they may drive the formation of lipid domains.
The three structural motifs of sterols that mediate their effects on membrane organization are the relatively rigid flat steroid ring structure present in all sterols, the hydrophilic 3Beta-Hydroxyl group present in ergosterol and cholesterol, which is able to interact with water at the bilayer/solvent interface, as well as the hydrocarbon chain at position 17 (Figure 11) (Dufourc, 2008). These structural parameters are thought to interact with different lipids in different ways, depending on the saturation state and length of the lipid acyl chain as well as the head group type (Dufourc, 2008).
Figure 11. The structural motifs of ergosterol that mediate its interactions with membranes. Structural features have been color coded; red depicts the steroid ring structure common to all sterols, green is the 3-Beta-Hydroxy group while blue shows the hydrocarbon chain at position 17.
OH
In order to understand the role of sterols in membranes, it is useful to consider a third type of lamellar phase not yet discussed; the gel (Lβ) phase. This lamellar phase is characteristic of saturated lipids with longer acyl chains and high TM’s (Goni & Alonso, 2009) and is
characterized by a very low degree of translational and rotational motion at lower
temperatures. This phase is not be to be confused with the Lo phase, where the degree of translational motion/diffusion is much higher. As the temperature of the environment approaches the Tm (melting temperature) for the lipid constituting the Lβ phase, the rise in entropic energy causes the acyl chains of these lipids to become disordered, and this phase transitions into the already discussed Ld phase. However, if we are to consider a mixture of 2 lipids of different Tm’s where both Lβ and Ld phases are present (i.e. the temperature is below the higher Tm but above the lower TM), then the addition of sterols to this system will
drastically change the organization of this membrane. Specifically, sterols will have a clear preference for the conformationally ordered lipid chains present in the Lβ phase, due to its flat and rigid steroid ring. Paradoxically, this high degree of order in the Lβ phase also counteracts the association of sterols due to relatively awkward shape of the sterol molecule, thereby giving preference to the less ordered acyl chains in the Lo phase, where it can intercalate between the disordered lipid chains. Interestingly, sterols seemed to have solved this paradox by inducing the formation of the Lo phase (Ipsen, Karlstrom, Mouritsen, Wennerstrom, & Zuckermann, 1987) (Figure 12), which can be thought of as an intermediate between the Lβ and Ld lamellar phases. In this complex scenario, the effects of sterol are twofold, depending on which of these two phases it associates with. Whenever sterols associate with the relatively disordered lipid chains, sterols cause the tighter arrangement of lipids by increasing the order of these chains via an increase in the proportion of trans acyl chain conformers. The conformation of an acyl chain relates to the various angles between two adjacent carbon chains and cholesterol essentially causes the ‘straightening’ of acyl chains, even when they are unsaturated. This in turn has the effect of decreasing the total surface area of an individual lipid leading to thickening of the membrane as well as increasing the packing between lipids leading to an overall decrease in fluidity and an increase in membrane stiffness. On the other hand, when the concentration of sterols increases and starts to associate with the Lβ phase, the association of
the sterol rings with the ordered chains of the Lβ phase has the opposite effect since this intercalation decondenses the acyl chains (or decreases the total surface area of a lipid molecule) and fluidizes the Lβ phase into an Lo phase. In biological terms, the implications of this are dramatic! Biological membranes contain lipids with high Tms, such as SLs, as well as lipids with much lower Tms. In this setting, membrane sterols have the ability to not only modulate the fluidity of the Ld phase, but also keep the high Tm lipids from “solidifying”
membranes, essentially acting as an anti-freeze agent (Laggner, 2007). However, the most remarkable aspect of the effect of sterols on membranes is the notion that the addition of a single lipid species can dramatically change the organization and function of the entire
membrane. Furthermore, due to the ability of sterols to modulate the membrane environment via their interactions with other lipid species, sterols have served as a paradigm to understand how membrane characteristics can be regulated. Because they exert their influence by
interacting with various lipids and essentially “filling” in the spaces between membrane lipids, they have helped in understanding how other membrane-associated molecules such as carotenoids (Manabe, Hirata, & Sugawara, 2019) and ceramides (Yu, Alterman, & Dobrowsky, 2005) affect the lateral organization of cellular membranes since they seem to exhibit some structural and functional similarities to sterols.
Figure 12. Cholesterol modulates the formation of Lo domains. The addition of cholesterol to an Ld-phase (black lipids; top left) or to an Lβ-phase (green lipids; middle) changes their physical characteristics and drives the formation of an Lo-phase. Note the effects of cholesterol on rotational and translational motions for each phase.
The roles of sphingolipids and membrane thickness in domain formation.
As was mentioned previously, the interaction affinities between different lipids and sterols not only depends on the sterols ability to associate with a given phase, but also depends on structural elements present on the lipid itself. Of particular interest is the structure of the lipid backbone. SLs, which are made of a sphingoid base backbone together with a very long chain fatty acid (VLCFA) have by far the longest acyl chains of any lipids present in the cell (Denic &
Weissman, 2007). It is thought that the ability of sterols to preferentially associate with SLs is due
to the fact that the longer acyl chains of SLs provide sterols a bigger hydrophobic platform for association (Ramstedt & Slotte, 2002; Rog, Pasenkiewicz-Gierula, Vattulainen, & Karttunen, 2009).Furthermore, SL acyl chains are not only longer than GPL acyl chains, but are also more saturated (and therefore straighter), which further stabilize sterol-SL interactions (Ramstedt &
Slotte, 2002). Lastly, the sphingoid backbones of SL contain both hydroxyl (OH-) and amine (NH3+) groups which can act as both hydrogen bond donors and acceptors with sterols (Rog et al., 2009). An instructive example of how these subtle differences impact the formation of lipid domains is the case of the two main sphingosine bases making up the SLs found in yeast; DHS and PHS, which differ in the presence of a single hydroxyl group at position 4 (Haak et al., 1997).
DHS, which is the precursor to ceramide and PHS, which is the precursor to phytoceramide, differ in their ability to modulate membrane behavior as phytoceramide is more disposed to form lipid domains than DHS-derived ceramide (Marques et al., 2015). Due to the presence of an extra hydroxyl group in phytoceramides, this lipid species ability to interact with other lipids, such as sterols, is greater than the non-hydroxylated version.
One particularly interesting aspect of SL structure relevant to this work is the role of the headgroup in SLs. Ceramides, which are known as the simplest SL due to the lack of a complex headgroup differ from more complex sphingolipids in their ability to modulate membrane properties. One striking difference between membrane-raft forming SLs such as sphingomyelin and ceramides has to do with their interactions with sterols and with the acyl chains of the bilayer. Because ceramides have much smaller headgroups than complex SLs, ceramides are able to behave like sterols in membranes, mainly by intercalating between different lipids and inducing a higher degree of membrane order. This effect is most likely due to the fact that ceramides, with their small headgroups, can reside in the spaces between lipids with much larger headgroups, effectively shielding their hydrophobic portions from the aqueous environment (Figure 13).
Figure 13. Sphingomyelin (green) has a big headgroup which prevents it from entering the inter-lipid space while ceramide (red) can.
In fact, one of the first observations regarding this peculiar behavior came from studies where sphingomyelinase (SMase) was added to membranes containing cholesterol/sphingomyelin-rich raft structures. It was observed that the in situ production of ceramides by SMase effectively destabilized these rafts by disrupting the cholesterol- sphingomyelin interactions (Bashkirov et al., 2008; Garcia-Saez, Chiantia, & Schwille, 2007). This has led to the proposal that ceramides, due to their physical properties reminiscent of sterols (small headgroup and extremely hydrophobic) have the tendency to intercalate between the acyl chains of lipids and compete for the same inter-lipid spaces as sterols. In support of this notion, various instances have been reported where the addition of ceramides to either pure phospholipid bilayers or to bilayer mixtures mimicking lipid rafts (usually cholesterol, sphingomyelin and PC) modulate the properties of membranes similarly to cholesterol. For example, the addition of ceramides, even at relatively low concentrations (3 mol%) to phospholipid bilayers increased the order of the acyl chains leading to an increase in thickness and rigidity of the membrane (Al Sazzad, Yasuda, Murata, & Slotte, 2017) and promoted the formation of Lβ ceramide-rich lipid domains. However, the addition of higher amounts of cholesterol to this type of mixture had been shown to destabilize these ceramide rich domains, but not to Lo domains (Castro, Silva, Fedorov, de Almeida, & Prieto, 2009). Specifically, in a 5:1 mixture of POPC and ceramide, an increase of cholesterol (up to 20 mol%) changed the properties of the disordered phase towards a more ordered domain without affecting the ceramide-rich gel phase (Lβ). However, when cholesterol levels increased above this threshold, the formation of three phases was observed: two ordered phases, presumably from POPC/Chol and POPC/Cer as well as a third gel phase, presumably from Cer/Chol or just Cer (Fidorra, Duelund, Leidy, Simonsen, & Bagatolli, 2006). This is in stark contrast to mixtures where complex sphingolipids,
rather than ceramide, are used. As previously noted, the addition of cholesterol to these mixtures destabilizes the Lβ gel phase into the Lo phase, which is not the case in Cer/GPL/chol ternary mixtures (Fidorra et al., 2006; Goni & Alonso, 2006). Thus, although ceramide and more complex SL share many common structural characteristics, the differences in their headgroup and shape underlie their differential interactions with other membrane lipids and support the idea that lipid domains containing ceramide may be markedly different from the lipid domains described by the lipid-raft hypothesis. This notion is motivated by experiments done on more complex 4- component lipid mixtures, where it has been shown that the effect of ceramide strongly depends not just on its concentration, but more importantly on the relative amounts of ceramide and cholesterol present in the membrane (Alonso & Goni, 2018; Goni & Alonso, 2009). At low and non-saturating concentrations of cholesterol, the addition of ceramide to a raft-mimicking ternary lipid mixture (SM/PC/cholesterol) not only displaced the raft-associated cholesterol from Lo domains, but also changed the physical properties of these phases giving rise to a third type of Lo phase dependent on ceramide concentration (albeit with different structural characteristics from the cholesterol-rich sphingomyelin ordered phase) (Chiantia, Kahya, Ries, & Schwille, 2006).
Thus, these subtle differences of chemical structure present across SLs allow the cell to regulate and modulate the properties of its membranes, such as their fluidity and lateral organization, by simply changing the relative amounts of certain lipids. This notion has been of particular interest in terms of studying membrane domains in living cells, since ceramide producing enzymes such as SMase at the plasma membrane could dramatically change the behavior and signaling activities of membranes by increasing the level of ceramides thereby driving membrane reorganization (Dinkla et al., 2012). Furthermore, great strides have been made in elucidating the various transport pathways for membrane lipids and it will be important to investigate the relative effect these processes have on the organization of cellular membranes through their modulation of organellar lipid levels. Perhaps a more interesting line of research would be to look at the effects of less studied lipids, such as ceramide, on SL and sterol-poor membranes, since it is clear that the ordering and domain forming effects of ceramides on membranes are different depending on the relative cholesterol and SL levels. Furthermore, because ceramides not only share some of the same characteristics of their more complex SL counterparts, but also
exhibit some of the same characteristics as sterols, even at low concentrations, it would be incredibly interesting to look at the effects of ceramide levels on non-plasma membrane membranes, such as the endoplasmic reticulum. For example, the ER of yeast is the main site of ergosterol and ceramide synthesis and it is clear that the cell spends considerable resources to efficiently export most of the ceramides and ergosterol from the ER (Schulz & Prinz, 2007).
However, it is also clear that ceramides dramatically change the properties of membranes and can potentially induce phase separation of small functional lipid domains at these membranes.
Thus, it would be interesting to not only investigate how the cell keeps this potentially damaging lipid at low levels in the ER but also to consider the possibility that ceramide levels may play an organizational role in these membranes. One may even envision a scenario where the physico- chemical properties of different membranes could be drastically modulated in response to certain stresses through the modulation of ceramide levels at these membranes. While this may seem like a far-fetched idea to the reader, I would just like to remind that there has been a recent surge of papers showing how whole cellular compartments drastically change their physical properties in response to stress, such as the bacterial cytoplasm (Parry et al., 2014).
Interestingly, the longer acyl chains of SLs may also drive the formation of membrane domains, not just through their preferential association with sterols, but also through the thermodynamic consequences associated with having longer acyl chains in a bilayer (Heberle et al., 2013). If one is to consider a lipid bilayer where two phases co-exist; a Ld phase consisting of unsaturated lipids with short acyl chains and a Lo phase consisting of longer saturated lipids, then the differences in the thickness of these two phases carries an energetic penalty by increasing the line tension profile of the membrane, which is defined as the energetic cost per unit length of the interfacial boundary line (i.e. along the surface of the bilayer; (Cheng & Smith, 2019)). This energetic cost is due to the hydrophobic mismatch of the two phases. Due to this energetic penalty, it is thermodynamically favorable for small Lo domains exhibiting a thicker membrane profile to coalesce into larger domains, since this reduces the line tension at the Lo/Ld interface (Garcia-Saez et al., 2007; Sezgin et al., 2017) (Figure 14). By coalescing smaller pre-existing ordered domains into larger ones, the total line tension of the whole membrane is effectively minimized, since the interface between these two phases are also minimized. In support of this
notion, it was found that the effective size of Lo domains strongly correlates to differences between domain thicknesses (Garcia-Saez et al., 2007). While this implies that it may be possible for the cell to regulate the sizes of lipid domains according to the acyl chains present in its lipids, it also suggests that a certain degree of membrane lateral organization could be driven by simply having a lipid repertoire with a high degree of acyl chain variability, since this has been shown to be enough to drive domain formation.
Figure 14.Hydrophobic missmatch organizes membranes by reducing line tension. If two lipids of different length (long lipid; red, short lipid; green) are in a mixture, then the hydrophobic missmatch between the two lipids generates line tension (black arrows). As this system approaches equilibrium, lipids are unmixed into larger domains which minimizes the total line tension of the membrane. The right hand side of the figure shows how this would look if we observe the bilayer from above.
short lipid short lipid long lipid
long lipid
reduction of line tension
In conclusion, the subtle structural differences present throughout the vast repertoire of membrane lipids are the basis for the remarkable behaviors that membranes exhibit. In the case of SLs and sterols, it is interesting to ponder whether or not these lipids have structurally co- evolved to bring about emergent phenomena, as is seen in the formation of lipid domains.
Furthermore, it is clear that SLs display a wide range of behaviors based on their chemical structure. A peculiar example of this is the case of ceramides, which not only have behaviors reminiscent of both sterols and complex SLs, but are also able to change the characteristics of lipid domains composed of SLs and sterols as well as forming their own phases with different physical characteristics. Lastly, common characteristics of these different lipid domains, such as their increased thickness, are a direct consequence of their composition, which can further influence the organization of the membrane at even larger scales since this is enough to thermodynamically drive domain formation. This last consideration is of particular importance in the coming section, since the same forces driving the formation of domains may also be important in determining the localization of other membrane components, such as proteins, to these domains.
How are proteins targeted to lipid domains?
Up until now, we have considered the various lipid-lipid interactions underlying the formation of lipid domains. Nonetheless, a second question of great importance is what determines that a given protein goes to these domains in the first place. Thus, the essence of this question concerns the protein-lipid interactions happening at the membrane, rather than lipid- lipid interactions. After all, the biological functions ascribed to the existence of lipid rafts/domains are defined by the ability of these domains to modulate the organization of other cellular factors, such as protein complexes present on the membrane. Furthermore, identifying the structural features and motifs that target a given membrane protein to a given lipid domain would also help in the development of tools and methodologies needed to study these domains in vivo, which at the moment is still lacking. Lastly, the characterization of the different features mediating lipid-protein interactions are one of the least understood phenomena in molecular biology (Corradi et al., 2019) and a fundamental understanding of these processes is crucial in