Magnetic Resonance Imaging of the Axial Skeleton in Patients With Spondyloarthritis: Distribution Pattern of Inflammatory and Structural Lesions
Volltext
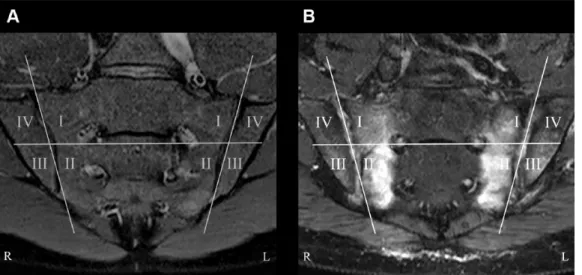
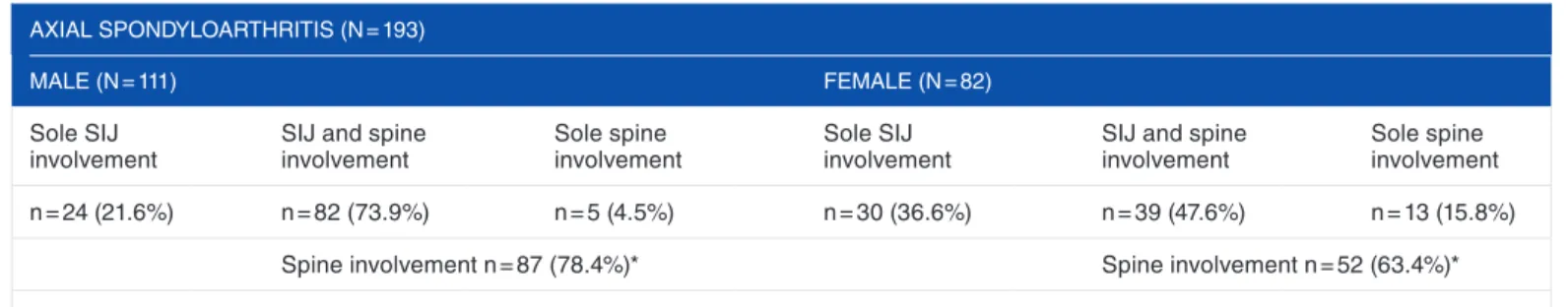
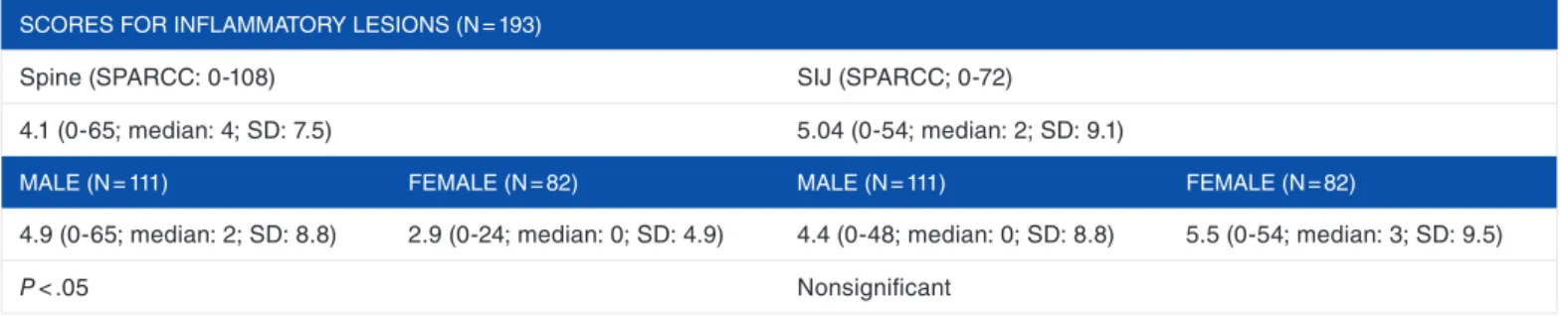
Abbildung
ÄHNLICHE DOKUMENTE
Percentage of current year earlywood vessel-elements connected to previous year latewood vessel-elements (A) and mean (±SD) flow (B) expressed as the proportion of axial flow in
Woodhams R, Matsunaga K, Iwabuchi K, Kan S, Hata H, Kuranami M, Watanabe M, Hayakawa K (2005) Diffusion-weighted imaging of malig- nant breast tumors: the usefulness of
Pearce RH (1991) Magnetic resonance imaging reflects the chemical changes of aging degeneration in the human inter- vertebral disc. Tertti M, Paajanen H, Laato
Pearce RH (1991) Magnetic resonance imaging reflects the chemical changes of aging degeneration in the human inter- vertebral disc. Tertti M, Paajanen H, Laato
chapter 4: How does the complex consistency of brain tissue, consisting of coupled pools of protons (e.g., myelin water – free water), affect the outcome of the
However, it is worth noting that the improvement in lesion visualization seen on combined pre- plus post-contrast images relative to pre-contrast images alone bears excellent
To investigate the response of the volume elements to the excitation detached from the signal of the falling motion, the relative phase ∆ϕ r with respect to a reference point in
This subset comprises volumetric MRI scans of the speaker’s vocal tract during sustained production of vowels and consonants, as well as dynamic mid- sagittal scans of