Zeitschrift für Kristallographie - New Crystal Structures 212, 271-272
© by R. Oldenbourg Verlag, München 1997
271
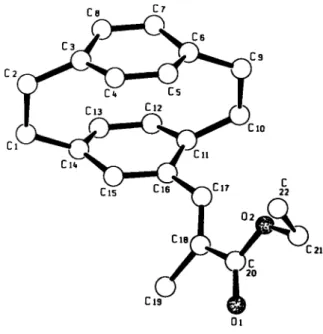
Crystal structure of iM-(2'-ethoxycarbonyl-2'-methyl)-ethenyl-[2.2]para- cyclophane, C22H24O2
B. Gollas, B. Speiser
Universität Tübingen, Institut für Organische Chemie, Auf der Morgenstelle 18, D-72076 Tübingen, Germany
J. Sieglen, J. Strähle and C. Maichle-Mössmer
Universität Tübingen, Institut für Anorganische Chemie, Auf der Morgenstelle 18, D-72076 Tübingen, Germany Received October 1, 1996, CSD-No. 402630
Table 1. Parameters used for the X-ray data collection
Source of material: The title compound was synthesized by Rieche formylation of [2.2]paracyclophane to give 4-foimyl-[2.2]paracy- clophane (see réf. 1) which in turn was treated with triethyl-2- phosphonopropionate in a Wittig reaction (see ref. 3).
Ethenyl-substituted cyclophanes have been synthesized by Hopf and co-workers (see réf. 1). We investigate electrochemical properties of these compounds in an attempt to produce oligo- and polymers by anodic side-chain polymerization (see ref. 2). The new title compound is a polar derivative of 4-vinyl[2.2]paracyclo- phane (see réf. 1). H atoms are calculated on ideal positions.
C 2 2 H 2 4 O 2 ,
monoclinic,
P\2\lc\(No. 14),
a=18.537(2) Â,
¿>=8.1021(5) Â, c =11.673(2) Â, β =87.25(1)°, V=1751.1 Â
3, Z=4, p
m=1 215 gem
-3, R(F) =0.062, R^F) =0.058.
Crystal: colorless, thin plate, size 0.45 χ 0.20 χ 0.05 mm
Wavelength: Cu Ka radiation (1.54184 Â)
μ; 56.04 cm"1
Diffractometer Enraf-Nonius CAD4
Scan mode: ω
Tnuasurement'· 291 Κ
20tnui: 130°
NCAAOum^: 1569
Criterion for /0: Λ> > 3 σ(/ο) N(param)rr/Uvj: 218
Table 2. Final atomic coordinates and displacement parameters (in À2)
Atom Site χ y ζ fiso
H(1A) 4e H(1B) 4e H(2A) H(2B) H(4) H(5) H(7) H(8) H(9A) H(9B) H(10A) 4e H(10B) 4e H(12) H(13) H(15) H(17) H(19A) 4e H(19B) 4e H(19C) 4e H(21A) 4e H(21B) 4e H(22A) 4e H(22B) 4e H(22C) 4e 4e 4e 4e 4e 4e 4e 4e 4e
0.9036(3) 0.9465(3) 1.0088(3) 0.9664(3) 0.8757(3) 0.7916(3) 0.8939(3) 0.9771(3) 0.7244(3) 0.7672(3) 0.7179(3) 0.6655(3) 0.8001(3) 0.8832(3) 0.7800(2) 0.6690(2) 0.5926(3) 0.6178(3) 0.6751(3) 0.5023(3) 0.5118(3) 0.5541(4) 0.6082(4) 0.6177(4)
0.0461(7) 0.1816(7) -0.0097(8) -0.1450(8) -0.3155(6) -0.3294(6) 0.0292(6) 0.0420(7) -0.1818(8) -0.0623(8) 0.1556(7) 0.0350(7) 0.3197(6) 0.3319(6) -0.0257(5) -0.1615(6) -0.0488(7) 0.1033(7) -0.0106(7) -0.4955(8) -0.4994(8) -0.7385(9) -0.6375(9) -0.6414(9)
0.4869(5 0.4164(5 0.3255(5 0.3968(5 0.2988(4 0.1616(4 -0.0122(4 0.1252(5 0.0181(5 -0.0659(5 0.0175(4 0.0855(4 0.1241(4 0.2621(4 0.4335(4 0.2350(4 0.5478(5 0.4732(5 0.5280(5 0.3641(7 0.2295(7 0.3050(7 0.3767(7 0.2426(7)
0.08542 0.08542 0.09571 0.09571 0.07183 0.07051 0.07705 0.08033 0.09335 0.09335 0.07648 0.07648 0.06716 0.07103 0.05694 0.05835 0.10923 0.10923 0.10923 0.11932 0.11932 0.17170 0.17170 0.17170
Table 3. Final atomic coordinates and displacement parameters (in Â2)
Atom Site χ y ζ U11 í/22 Uj3 Un Un Un O(l) 4e 0.5372(3) -0.2745(6) 0.4707(4) 0.146(4) 0.131(4) 0.128(4) -0.065(3) 0.072(3) -0.018(3) 0(2) 4e 0.5828(2) -0.3497(5) 0.3025(4) 0.083(3) 0.079(3) 0.112(3) -0.035(2) 0.025(2) -0.021(3) C(l) 4e 0.9165(3) 0.0844(7) 0.4099(5) 0.054(3) 0.091(4) 0.069(3) -0.004(3) -0.009(2) -0.016(3)
272
[2.2]Paracyclophane Table 3. (Continued)Atom Site X y ζ Un ί/22 t/33 ί/12 ί/13 ί/23
C(2) Ae 0.9610(3) -0.0523(8) 0.3453(5) 0.063(3) 0.098(5) 0.080(4) 0.018(3) -0.021(3) -0.012(3) C(3) 4e 0.9281(2) -0.1133(6) 0.2379(4) 0.050(3) 0.058(3) 0.062(3) 0.009(2) 0.001(2) -0.010(3) C(4) Ae 0.8763(3) -0.2371(6) 0.2405(4) 0.069(3) 0.048(3) 0.062(3) 0.012(3) 0.004(3) 0.002(3) C(5) 4e 0.8258(3) -0.2454(6) 0.1582(4) 0.064(3) 0.049(3) 0.063(3) -0.000(2) 0.003(2) -0.010(3) C(6) 4e 0.8252(3) -0.1302(6) 0.0701(4) 0.069(3) 0.058(3) 0.046(3) 0.010(3) -0.004(2) -0.011(3) C(7) 4e 0.8867(3) -0.0319(6) 0.0548(4) 0.077(4) 0.063(3) 0.052(3) -0.001(3) 0.011(3) 0.002(3) C(8) 4e 0.9365(3) -0.0245(7) 0.1372(5) 0.051(3) 0.068(4) 0.081(4) -0.004(3) 0.011(3) -0.010(3) C(9) 4e 0.7562(3) -0.0867(8) 0.0144(5) 0.093(4) 0.090(4) 0.053(3) 0.008(3) -0.028(3) -0.013(3) C(10) 4e 0.7159(3) 0.0639(7) 0.0710(4) 0.067(3) 0.070(3) 0.055(3) -0.003(3) -0.014(2) 0.018(3) C(ll) Ae 0.7466(2) 0.1186(6) 0.1817(4) 0.049(2) 0.046(3) 0.050(3) 0.006(2) -0.005(2) 0.005(2) C(12) Ae 0.7988(3) 0.2405(6) 0.1819(4) 0.063(3) 0.046(3) 0.059(3) -0.001(2) -0.000(2) 0.011(2) C(13) Ae 0.8489(3) 0.2481(6) 0.2647(4) 0.055(3) 0.050(3) 0.072(4) -0.010(2) 0.008(2) -0.012(3) C(14) Ae 0.8488(2) 0.1319(6) 0.3523(4) 0.051(3) 0.062(3) 0.046(3) -0.001(2) -0.002(2) -0.014(3) C(15) Ae 0.7869(2) 0.0359(5) 0.3667(4) 0.049(2) 0.052(3) 0.041(2) -0.000(2) 0.001(2) -0.004(2) C(16) Ae 0.7349(2) 0.0283(5) 0.2846(4) 0.041(2) 0.047(3) 0.042(2) -0.003(2) -0.003(2) -0.002(2) C(17) Ae 0.6759(2) -0.0929(6) 0.2974(4) 0.051(2) 0.048(3) 0.048(3) -0.002(2) -0.007(2) 0.004(2) C(18) Ae 0.6316(2) -0.1144(6) 0.3889(4) 0.049(2) 0.048(3) 0.051(3) 0.005(2) 0.001(2) 0.003(2) C(19) Ae 0.6290(3) -0.0081(7) 0.4939(5) 0.073(3) 0.073(4) 0.070(3) -0.005(3) 0.015(3) -0.004(3) Q20) Ae 0.5794(3) -0.2531(7) 0.3919(5) 0.055(3) 0.061(3) 0.076(4) -0.007(2) 0.012(3) 0.006(3) C(21) Ae 0.5375(3) -0.4968(8) 0.2999(7) 0.079(4) 0.072(4) 0.147(6) -0.032(4) 0.005(4) -0.011(4) C(22) Ae 0.5832(4) -0.6406(9) 0.3066(7) 0.118(6) 0.083(5) 0.145(7) -0.020(5) -0.034(5) 0.019(5)
Acknowledgments. We thank V. Boekelheide, Eugene, Oregon, USA, for providing us with [2.2]paracyclophane as starting material and die Deutsche Forschungsgemeinschañ and the Fond der Chemischen Industrie for financial support B.S. thanks the DFG for the award of a Heisenberg fellowship.
References
1. Herrmann, E.: Synthese und Polymerisation von Vinylderivaten des [2.2]Paracyclophans. Dissertation, Technische Hochschule Braun- schweig, Germany 1990.
2. GoUas, B.; Hesse, L; Lötz, R.; Speiser, Β.: Electrochemical oxidation of Ethenyl-substituted Cyclophanes. 4-Ethenyl-[2.2]-paracyclophane: Cy- clic Voltammetry, Electrosynthesis of Polymers, Analysis by Liquid Chro- matography, and Mass Spectrometry. In preparation.
3. Gallagher jr., G.; Webb, R. L.: Tetrasubstituted Acrylates: The Wittig- Horner Reaction of Ketones with Triethyl α-phosphonopropionate. Syn- thesis (1974) 122-124.
4. Sheldrick, G. M.: Phase annealing in SHELX-90: Direct methods for larger structures. Acta Crystallogr. A46 (1990) 467-473.
5. Sheldrick, G.M.: SHELXL-93, a program for refining crystal structures.
University of Göttingen, Germany 1993.