Osmoregulation of the proU operon at a post-transcriptional level in Escherichia coli
Volltext
Abbildung


ÄHNLICHE DOKUMENTE
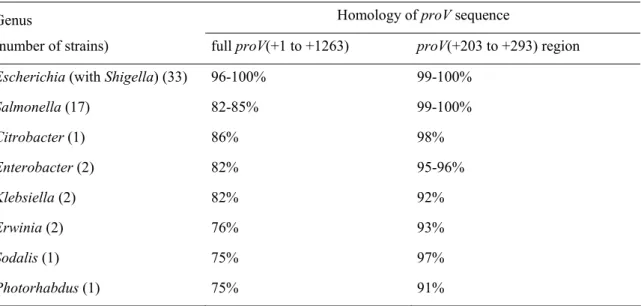
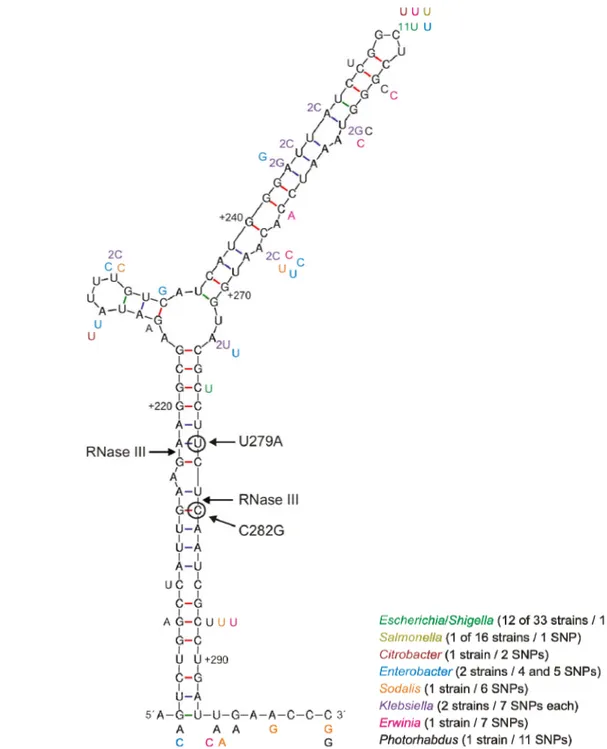
Based on the data obtained from the csrA::Tn5 strain, it is tempting to conjecture that under conditions where CsrA activity is inhibited, at the beginning of stationary phase for
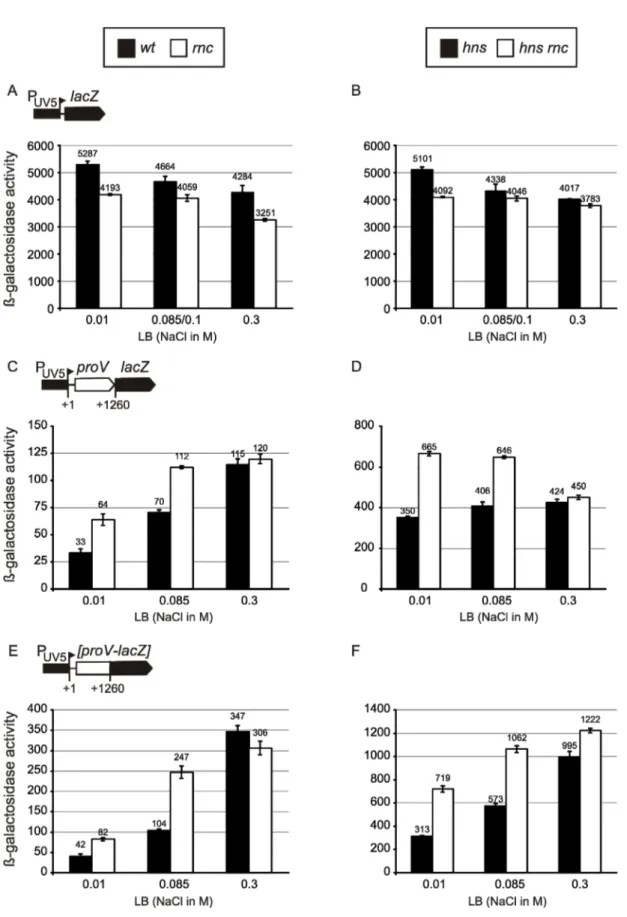
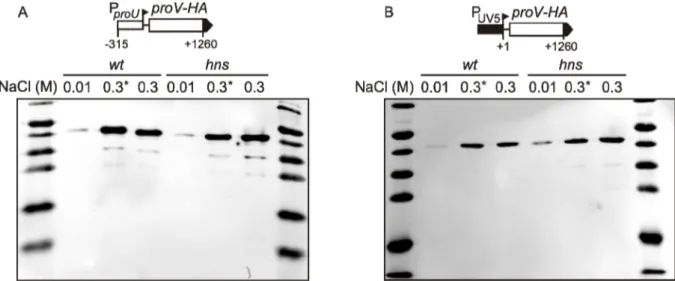
is appropriate for studying specific parts of transcription units like different ribosome binding site (RBS) or promoters, but is limited for investigations of effects on
This structure, which represents a kinetically trapped reaction intermediate of DsbB, contains a charge-transfer complex between the Cys44 of DsbB and ubiquinone Q8 in its active
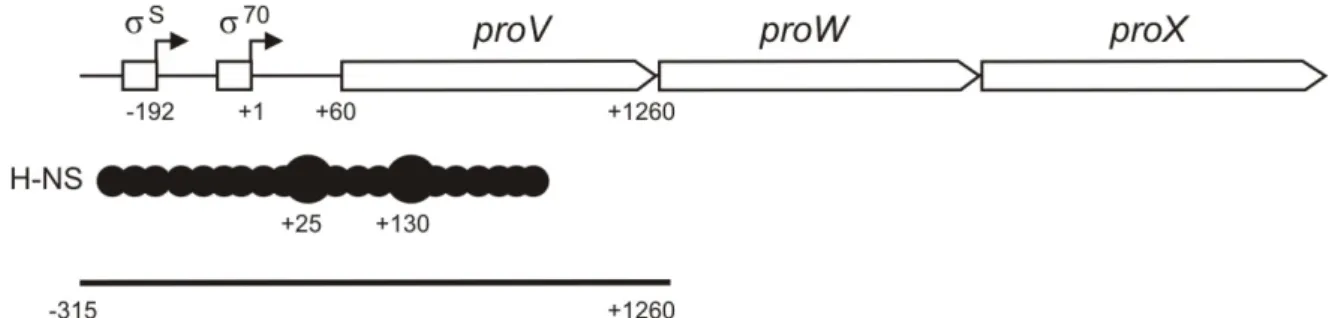
To identify important regulatory motifs in the promoter region, mutations were introduced into the conserved elements region by PCR (primers.. TATA boxes, CAAT and TAAG motifs
Fatty acid incubation and analysis Yeast cultures of the only effective transformation process with HO-poly-KanMX4-HO + GPD prom + fat-1t + term as demonstrated by repeated positive
With the means of HDACs and SMRT inhibitors the function of the recruited complex was verified and a novel mechanism of target gene dependent VDR- mediated
Photo- graphs were taken from time lapse microscopy during microfluidic per- fusion cultivation (A) and microscale batch cultivation (B) using LB medium supplemented with 100 mM (A)
The following facts support this hypothesis: (i) the Sim protein is synthesized as a precursor with a hydrophobic leader sequence of 20 amino acid resi- dues,