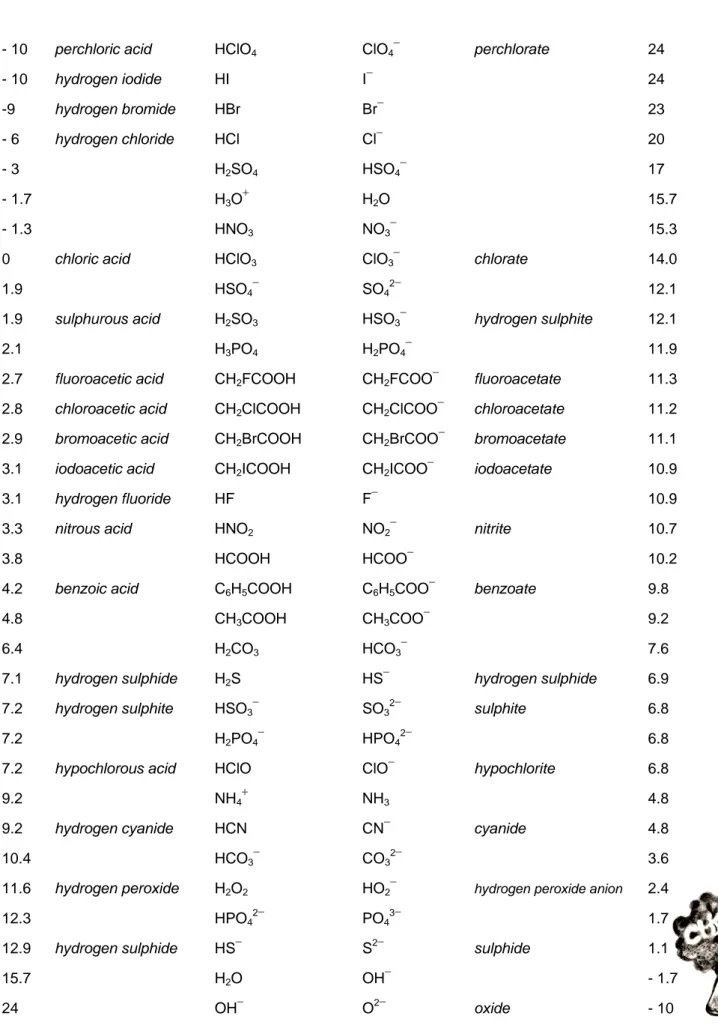
Table of Conjugate Acid-Base Pairs
pK
a- 10 - 10 -9 - 6 - 3 - 1.7 - 1.3 0 1.9 1.9 2.1 2.7 2.8 2.9 3.1 3.1 3.3 3.8 4.2 4.8 6.4 7.1 7.2 7.2 7.2 9.2 9.2 10.4 11.6 12.3 12.9 15.7 24
perchloric acid hydrogen iodide hydrogen bromide hydrogen chloride
chloric acid
sulphurous acid
fluoroacetic acid chloroacetic acid bromoacetic acid iodoacetic acid hydrogen fluoride nitrous acid
benzoic acid
hydrogen sulphide hydrogen sulphite
hypochlorous acid
hydrogen cyanide
hydrogen peroxide
hydrogen sulphide
acid
HClO4
HI HBr HCl H2SO4
H3O HNO3
HClO3
HSO4
H2SO3
H3PO4
CH2FCOOH CH2ClCOOH CH2BrCOOH CH2ICOOH HF
HNO2
HCOOH C6H5COOH CH3COOH H2CO3
H2S HSO3
H2PO4
HClO NH4
HCN HCO3
H2O2
HPO4 2
HS H2O OH
conj. base
ClO4
I Br Cl HSO4
H2O NO3
ClO3
SO4 2
HSO3
H2PO4
CH2FCOO CH2ClCOO CH2BrCOO CH2ICOO F
NO2
HCOO C6H5COO CH3COO HCO3
HS SO3
2
HPO4 2
ClO NH3
CN CO3
2
HO2
PO4 3
S2 OH O2
perchlorate
chlorate
hydrogen sulphite
fluoroacetate chloroacetate bromoacetate iodoacetate
nitrite
benzoate
hydrogen sulphide sulphite
hypochlorite
cyanide
hydrogen peroxide anion
sulphide
oxide
pK
b24 24 23 20 17 15.7 15.3 14.0 12.1 12.1 11.9 11.3 11.2 11.1 10.9 10.9 10.7 10.2 9.8 9.2 7.6 6.9 6.8 6.8 6.8 4.8 4.8 3.6 2.4 1.7 1.1 - 1.7 - 10