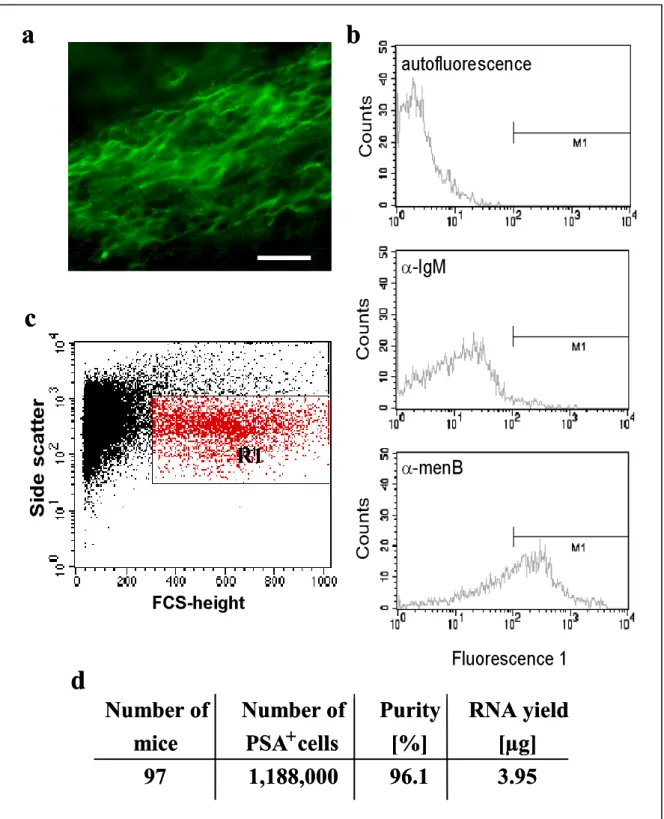
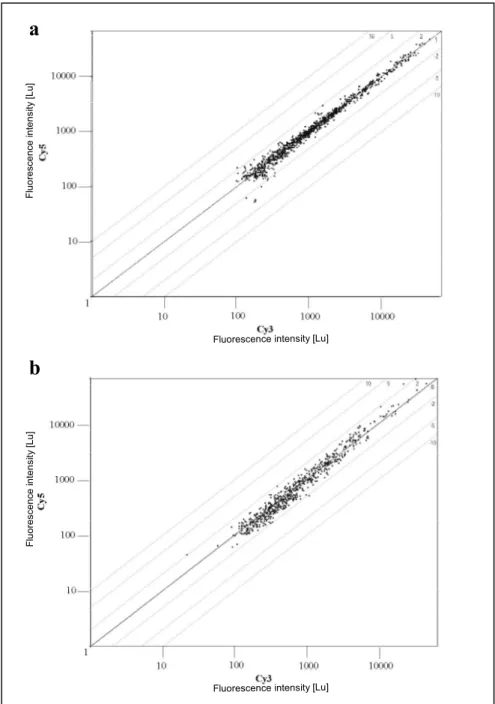
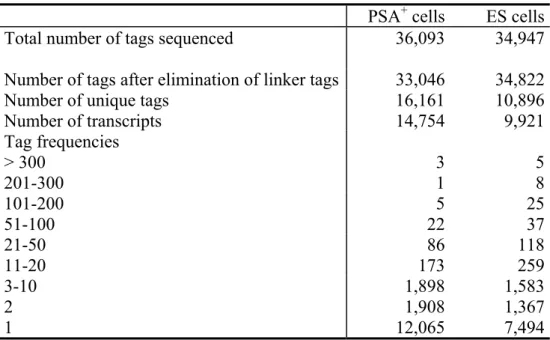
Gene expression analysis of neuronal precursors from adult mouse brain and differential screen for neural stem cell markers
Volltext
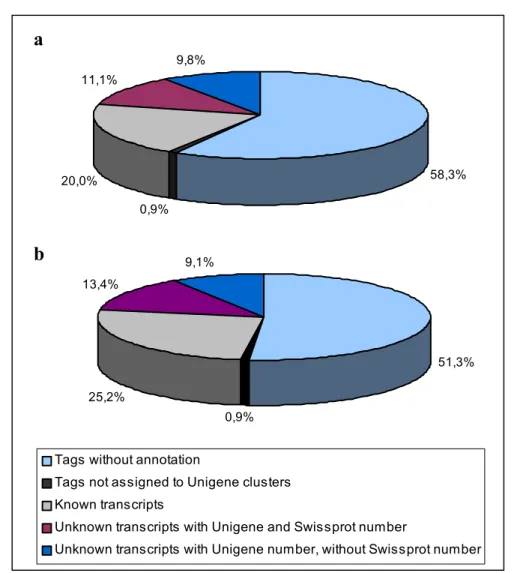
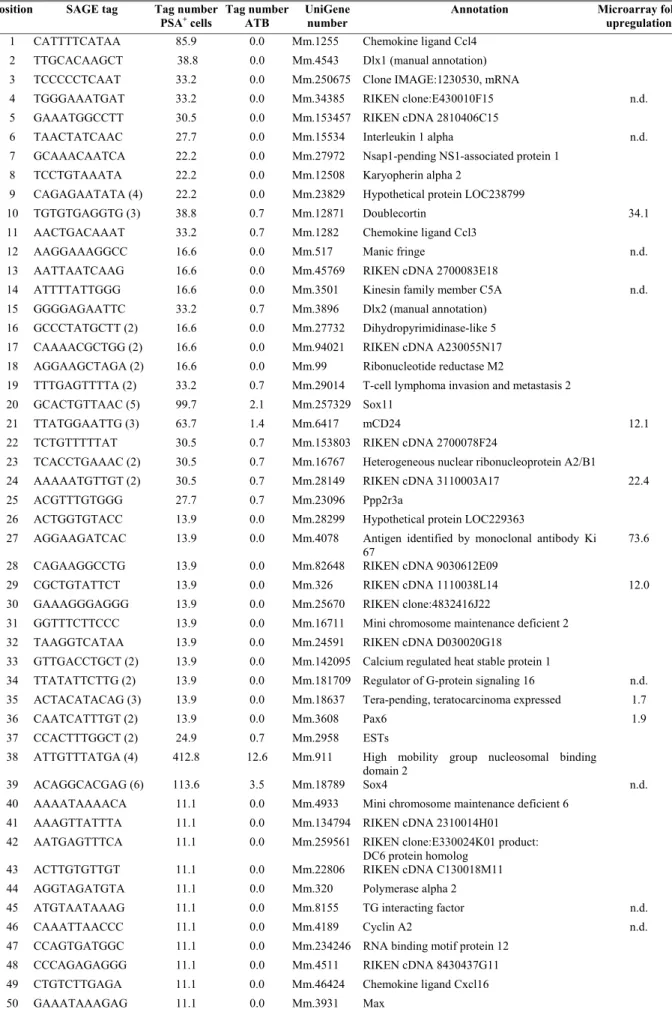
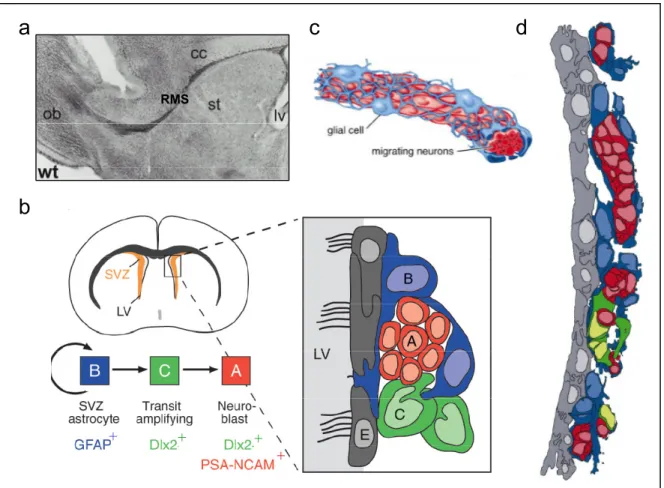
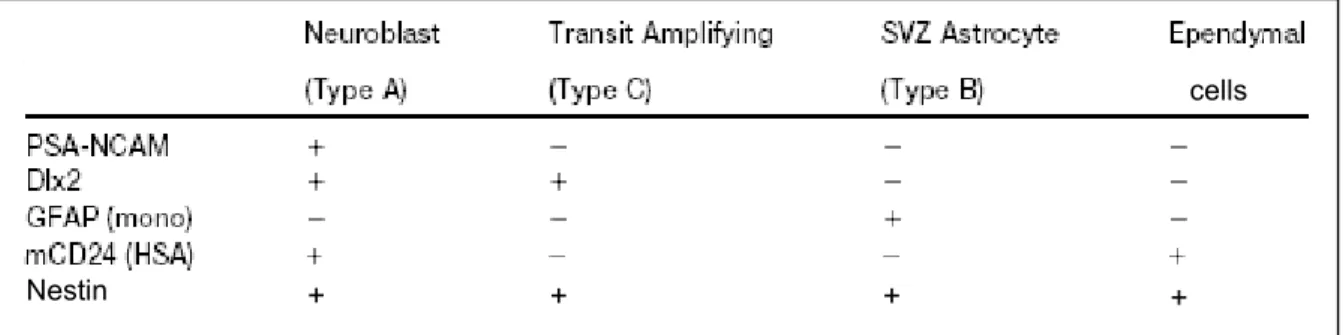
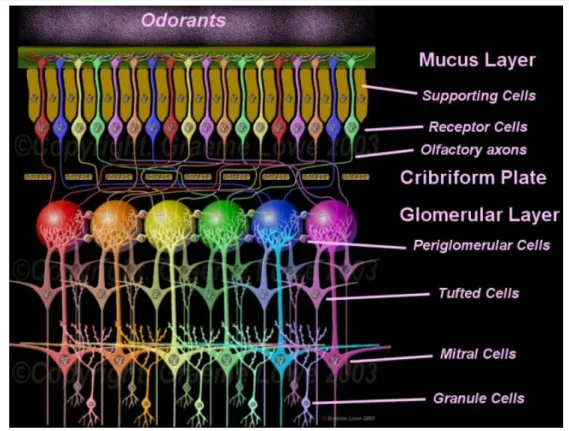
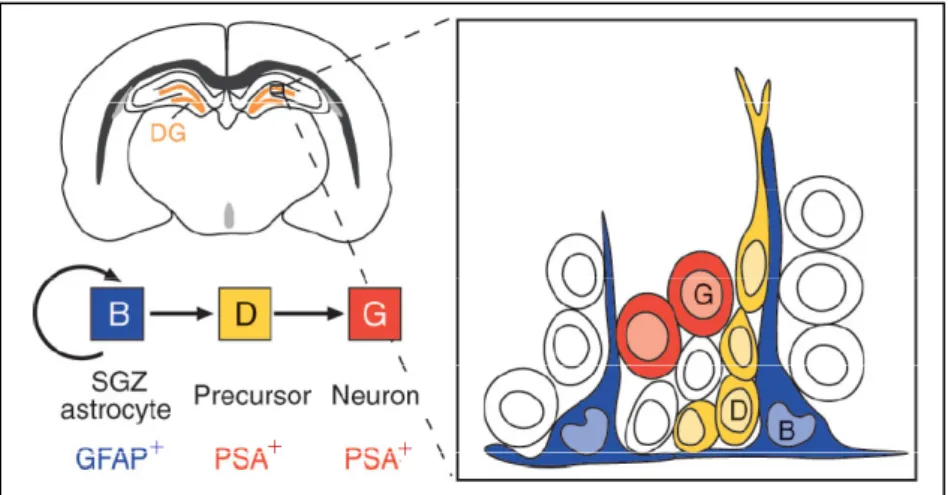
Abbildung
ÄHNLICHE DOKUMENTE
Representative RNAscope micrographs from a 3-month-old male C57BL/6 mouse probed for Gdf11 (white), probed for a cell specific marker (pink or green), and stained with DAPI
Because the Mecp2 -/y mice showed a significant increase in Trh compared to wt mice in almost all brain areas analyzed and the TRH function depends on the
By miRNA profiling of FACS-purified cells of the early V-SVZ lineage, the Doetsch group found the miR-17~92 cluster to be significantly upregulated in activated NSCs (aNSCs)
The current PhD thesis contributes to our understanding of the relation between ageing, the efficiency of attentional functions and their neural
For further analyses, it would be interesting to see whether the usage of a different inducible transgenic mouse lines in similar clonal lineage analysis, for example
Blocking OFF Pathway Tm Cells Reduces Responses of Lobula Plate Tangential Cells Specifically to OFF Edges The response properties of Drosophila lobula plate tangential cells have
By using MJD- patient-specific induced pluripotent stem cell-derived neural stem cells, our group found a possible mechanism for aggregate formation and why neurons
Five new microsatellite markers at candidate gene loci for litter size have been identified based on partial sequence analysis of porcine PAC and BAC clones containing the respective