Year 9 Chemistry – Worksheet 7
2.1 Structure of the electron shell
The first electron ionization energy is the energy needed to remove one (negatively charged) electron from the electrostatic attraction of the (positive) nucleus.
This energy generally increases along the second row of the periodic table from lithium (Li) to fluorine (F) because of the higher number of protons results in a stronger attraction of the electrons. This stronger attraction also causes the electron shell of the atoms to shrink from lithium to fluorine. This decrease in size of the electron shell, bringing the electrons closer to the nucleus, increases the attraction between them.
Further ionization energy of several elements
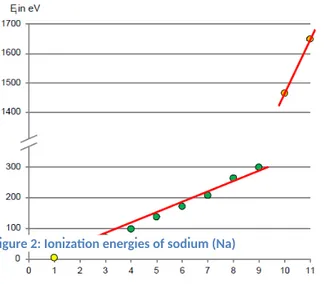
Take a look at the energy needed to remove all electrons from one atom type (three different examples). Come up with answers to the following questions:
a) Why does the ionization energy always increase when removing further electrons?
b) The electron shell of an atom is often split up into groups of electrons, called shells. How many shells does each of the
atoms have?
c) How many electrons does the first shell hold (closest to nucleus), how many does the second shell hold?
Figure 1: Ionization energies of neon (Ne)
Figure 2: Ionization energies of sodium (Na)
Figure 3: Ionization energies of magnesium (Mg)
Year 9 Chemistry – Worksheet 7
Extra task: Use the blank chart on the reverse to draw what you expect an ionization energy diagram for aluminum (Al) to look like!
Year 9 Chemistry – Worksheet 7
Figure 4: Ionization energies of aluminum (Al)
Year 9 Chemistry – Worksheet 7
Explanation of ionization energies
The explanation for the steadily __________________ ionization energies (e.g. from electrons 1-8 in neon) is that with each electron removed, the number of protons in comparison to electrons
_______________________________. Therefore the attraction of the nucleus is split up between ______________________ electrons. Because of this the attraction of each single electron by the nucleus is ____________________.
But when comparing electrons 1-8 and 9-10 in neon, the steady increase of ionization energy is _________________. Also there is a large ____________________ in the ionization energies when comparing electrons 8 and 9. These two observations show that there are different
___________________ of attraction when it comes to the electrons. When observing all of the elements on the reverse side of the worksheet, significantly _________________ energy is required to remove the last two electrons from the atom compared to earlier ones, so the attraction by the nucleus has to be a lot _____________________ here.