Zeitschrift fur Kristallographie - New Crystal Structures 213, 185-187 185
© by R. Oldenbourg Verlag, München 1998
Crystal structure of 4-(5,6-bis[isothiocyanato]-3,5-di-íe/t-butyl-4-oxo-2-cy- clohexadienylidene)-2,6-di-í^/t-butyl-2,5-cyclohexadienone, C30H40N2O2S2
Α. Rieker, W. Winter
Universität Tübingen. Institut für Organische Chemie. Auf der Morgenstelle 18. D-72076 Tübingen, Germany
W. Hiller and M. Neumayer
Technische Universität München, Anorganisch-Chemisches Institut, Lichtenbergstraße 4, D-85747 Garching, Germany
Received March 3, 1997, CSD-No. 405516
C20 C36
C34
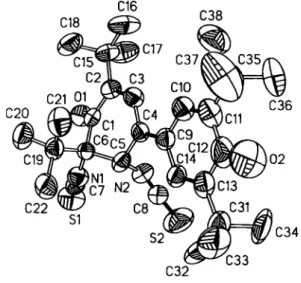
C32 C33 F i g . l . ORTEPplot.
Source of material: The compound was obtained by oxidation of 2,6-di-fórí-butyl-4-thiocyanatophenol in benzene with 2,6-di- fó/t-butyl-4-cyanophenoxyl or alkaline КзРе(СК)б as a by-pro- duct [4% yield]. At about458 К it decomposes witìi the loss of two tert-butyl groups to form a bis(benzoxazolthione) (see ref. 1).
The first isolatable product of the oxidative coupling sequence of 2,6-di-íerí-butyl-4-thiocyanatophenol is an asymmetric dihydro- p-diphenoquinone with two isothiocyanato groups at the sp^
carbon atoms. 2,6-Di-tórt-butyl-4-thiocyanatophenol did not give simple dehydrodimers (see refs. 1-4) on oxidative coupling but, surprisingly, both thiocyanato groups rearranged to the isothio- cyanato groups with a simultaneous change in position to give the title compound. An analysis of the ring puckering (see refs. 5-6) of CI to C6 reveals an envelope conformation ^ (see ref. 7) with the puckering amplitude Q = 0.419(2) Â and angles θ == 130.1 ( 1 )°
and φ = 115.0(1)°. The cyclohexadienone ring (C9 to С14) is slightly twisted against the best plane CI to C6 with an angle of 15.5(1)°. Another crystal structure with the same cyclohexenone motif, 2,6-di-iert-butyl-4-hydroxy-4,5-diphenylcyclohex-2-en- 1-one, showed a completely different geometry, i.e. an twist boat conformation (see ref. 8).
+47.7 -3.18
Fig. 2. Conformation and twsion angles of the title compound.
C3oH4oN2C)2Si orthorhombic, Pbca (No. 61), α =12.391(2) Â,
¿>=21.546(4) Â, с =23.133(5) Â, У=6176.0АЛ Z=8, =0.056,
Table 1. Parameters used for the X-ray data collection
Crystal: red, laismatic, size 0.2S χ 0.25 χ 0.30 mm Wavelength: Mo Ka radiation (0.71073 À)
μ: 1.99 cm"'
Difliractometer: Enraf-Nonius CAD4
Scan mode: ω
Tmeasurement'· 293 К
2втах: 40»
ЩНк1)итяие·· 2861
Criterion for Fo. f o > 4 o ( f „ ) N(param)rçffMrf: 338
Programs: SIR-92, SHELXL-93 (see refs. 9-13)
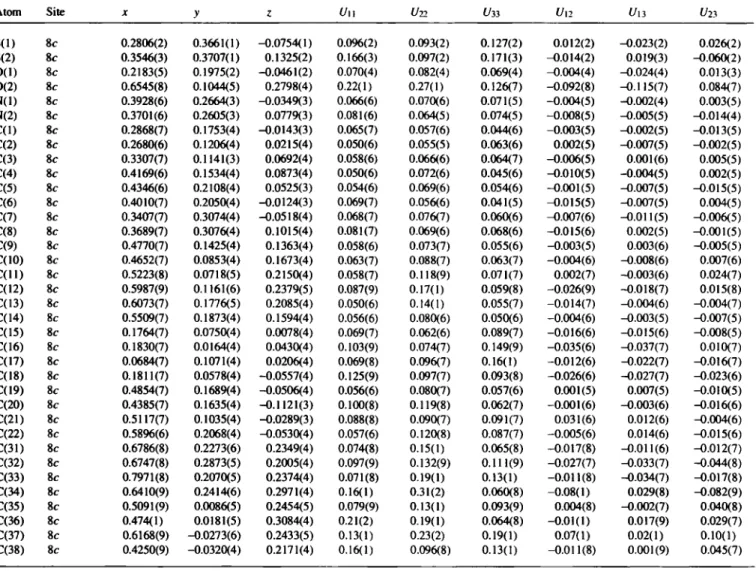
Table 2. Final atomic coordinates and displacement parameters (in Â^)
Atom Site X У ζ í/iso
H(3) 8c 0.3157(7) 0.0803(3) 0.0928(4) 0.075 H(5) 8c 0.5110(6) 0.2222(4) 0.0546(3) 0.071 H(10) Sc 0.4159(7) 0.0562(4) 0.1537(4) 0.085 H(14) 8c 0.5605(7) 0.2245(4) 0.1397(4) 0.074 H(16A) 8c 0.251(2) -0.003(2) 0.036(2) 0.163 H(I6B) 8c 0.125(3) -0.010(1) 0.032(2) 0.163 H(I6C) 8c 0.177(5) 0.0263(5) 0.0833(5) 0.163 H(17A) 8c 0.060(2) 0.143(2) -0.003(2) 0.164 H(I7B) 8c 0.067(2) 0.120(3) 0.0602(8) 0.164 H(17C) 8c 0.0103(8) 0.079(1) 0.014(3) 0.164
186
C 3 0 H 4 0 N 2 O 2 S 2Table 2. (Continued) Table 2. (Continued)
Atom Site X y с t/i«, Atom Site X y с f/iSO
H(18A) 8c 0.171(5) 0.0944(6) -0.0788(4) 0.158 H(33A) 8c 0.839(1) 0.239(2) 0.255(3) 0.193 H(18B) 8c 0.125(3) 0.029(2) -0.0644(7) 0.158 H(33B) 8c 0.823(2) 0.200(3) 0.1989(5) 0.193 H(18C) 8c 0.250(2) 0.040(3) -0.0643(7) 0.158 H(33C) 8c 0.803(1) 0.170(2) 0.260(3) 0.193 H(20A) 8c 0.417(4) 0.2039(5) -0.124(1) 0.140 H(34A) 8f 0.689(5) 0.271(4) 0.315(2) 0.266 H(20B) 8c 0.377(3) 0.137(2) -0.1115(6) 0.140 H(34B) 8<- 0.641(8) 0.204{ 1 ) 0.319(1) 0.266 H(20C) 8c 0.492(2) 0.147(3) -0.1375(6) 0.140 H(34C) 8c 0.569(3) 0.258(5) 0.2962(5) 0.266 H(21A) 8c 0.541(4) 0.1059(4) 0.010(1) 0.135 H(36A) 8f 0.408(4) 0.041(4) 0.3093(4) 0.231 H(21B) 8c 0.564(3) 0.085(1) -0.053(1) 0.135 H(36B) 8c 0.529(3) 0.040(4) 0.329(1) 0.231 H(21C) 8c 0.447(1) 0.0789(8) -0.028(2) 0.135 H(36C) 8f 0.463(7) -0.0216(5) 0.326( 1 ) 0.231 H(22A) 8c 0.5742(9) 0.2476(9) -0.067(2) 0.132 H(37A) 8c 0.668(3) -0.007(3) 0.268(3) 0.276 H(22B) 8c 0.640(2) 0.187(1) -0.077(2) 0.132 H(37B) 8ί· 0.644(4) -0.026(4) 0.205(1) 0.276 H(22C) 8c 0.620(2) . 0.210(2) -0.0149(5) 0.132 H(37C) 8c 0.605(2) -0.068(1) 0.256(4) 0.276 H(32A) 8c 0.722(4) 0.317(1) 0.218(2) 0.170 H(38A) 8c 0.421(4) -0.070(1) 0.237(2) 0.194 H(32B) 8c 0.602(1) 0.303(2) 0.200(2) 0.170 H(38B) 8c 0.445(3) -0.038(3) 0.178(1) 0.194 H(32C) 8c 0.698(5) 0.2795(8) 0.1616(9) 0.170 H(38C) 8c 0.356(1) -0.011(2) 0.219(3) 0.194
Table 3. Final atomic coordinates and displacement parameters (in Â^)
Atom Site X ζ ü l l Un t/33 (/12 и и f23
S(l) 8c 0.2806(2) 0.3661(1) -0.0754(1) 0.0%(2) 0.093(2) 0.127(2) 0.012(2) -0.023(2) 0.026(2) S(2) 8c 0.3546(3) 0.3707(1) 0.1325(2) 0.166(3) 0.097(2) 0.171(3) -0.014(2) 0.019(3) -0.060(2) 0(1) 8c 0.2183(5) 0.1975(2) -0.0461(2) 0.070(4) 0.082(4) 0.069(4) -0.004(4) -0.024(4) 0.013(3) 0(2) 8c 0.6545(8) 0.1044(5) 0.2798(4) 0.22(1) 0.27(1) 0.126(7) -0.092(8) -0.115(7) 0.084(7) N(l) 8c 0.3928(6) 0.2664(3) -0.0349(3) 0.066(6) 0.070(6) 0.071(5) -0.004(5) -0.002(4) 0.003(5) N(2) 8c 0.3701(6) 0.2605(3) 0.0779(3) 0.081(6) 0.064(5) 0.074(5) -0.008(5) -0.005(5) -0.014(4) C d ) 8c 0.2868(7) 0.1753(4) -0.0143(3) 0.065(7) 0.057(6) 0.044(6) -0.003(5) -0.002(5) -0.013(5) C(2) 8c 0.2680(6) 0.1206(4) 0.0215(4) 0.050(6) 0.055(5) 0.063(6) 0.002(5) -0.007(5) -0.002(5) C(3) 8c 0.3307(7) 0.1141(3) 0.0692(4) 0.058(6) 0.066(6) 0.064(7) -0.006(5) 0.001(6) 0.005(5) C(4) 8c 0.4169(6) 0.1534(4) 0.0873(4) 0.050(6) 0.072(6) 0.045(6) -0.010(5) -0.004(5) 0.002(5) C(5) 8c 0.4346(6) 0.2108(4) 0.0525(3) 0.054(6) 0.069(6) 0.054(6) -0.001(5) -0.007(5) -0.015(5) C(6) 8c 0.4010(7) 0.2050(4) -0.0124(3) 0.069(7) 0.056(6) 0.041(5) -0.015(5) -0.007(5) 0.004(5) C(7) 8c 0.3407(7) 0.3074(4) -0.0518(4) 0.068(7) 0.076(7) 0.060(6) -0.007(6) -0.011(5) -0.006(5) C(8) 8c 0.3689(7) 0.3076(4) 0.1015(4) 0.081(7) 0.069(6) 0.068(6) -0.015(6) 0.002(5) -0.001(5) C(9) 8c 0.4770(7) 0.1425(4) 0.1363(4) 0.058(6) 0.073(7) 0.055(6) -0.003(5) 0.003(6) -0.005(5) C(10) 8c 0.4652(7) 0.0853(4) 0.1673(4) 0.063(7) 0.088(7) 0.063(7) -0.004(6) -0.008(6) 0.007(6) C ( l l ) 8c 0.5223(8) 0.0718(5) 0.2150(4) 0.058(7) 0.118(9) 0.071(7) 0.002(7) -0.003(6) 0.024(7) C(12) 8c 0.5987(9) 0.1161(6) 0.2379(5) 0.087(9) 0.17(1) 0.059(8) -0.026(9) -0.018(7) 0.015(8) C(13) 8c 0.6073(7) 0.1776(5) 0.2085(4) 0.050(6) 0.14(1) 0.055(7) -0.014(7) -0.004(6) -0.004(7) C(14) 8c 0.5509(7) 0.1873(4) 0.1594(4) 0.056(6) 0.080(6) 0.050(6) -0.004(6) -0.003(5) -0.007(5) C(15) 8c 0.1764(7) 0.0750(4) 0.0078(4) 0.069(7) 0.062(6) 0.089(7) -0.016(6) -0.015(6) -0.008(5) C(16) 8c 0.1830(7) 0.0164(4) 0.0430(4) 0.103(9) 0.074(7) 0.149(9) -0.035(6) -0.037(7) 0.010(7) C(17) 8c 0.0684(7) 0.1071(4) 0.0206(4) 0.069(8) 0.096(7) 0.16(1) -0.012(6) -0.022(7) -0.016(7) C(18) 8c 0.1811(7) 0.0578(4) -0.0557(4) 0.125(9) 0.097(7) 0.093(8) -0.026(6) -0.027(7) -0.023(6) C(19) 8c 0.4854(7) 0.1689(4) -0.0506(4) 0.056(6) 0.080(7) 0.057(6) 0.001(5) 0.007(5) -0.010(5) C(20) 8c 0.4385(7) 0.1635(4) -0.1121(3) 0.100(8) 0.119(8) 0.062(7) -0.001(6) -0.003(6) -0.016(6) C(21) 8c 0.5117(7) 0.1035(4) -0.0289(3) 0.088(8) 0.090(7) 0.091(7) 0.031(6) 0.012(6) -0.004(6) C(22) 8c 0.5896(6) 0.2068(4) -0.0530(4) 0.057(6) 0.120(8) 0.087(7) -0.005(6) 0.014(6) -0.015(6) C(31) 8c 0.6786(8) 0.2273(6) 0.2349(4) 0.074(8) 0.15(1) 0.065(8) -0.017(8) -0.011(6) -0.012(7) C(32) 8c 0.6747(8) 0.2873(5) 0.2005(4) 0.097(9) 0.132(9) 0.111(9) -0.027(7) -0.033(7) -0.044(8) C(33) 8c 0.7971(8) 0.2070(5) 0.2374(4) 0.071(8) 0.19(1) 0.13(1) -0.011(8) -0.034(7) -0.017(8) C(34) 8c 0.6410(9) 0.2414(6) 0.2971(4) 0.16(1) 0.31(2) 0.060(8) -0.08(1) 0.029(8) -0.082(9) C(35) 8c 0.5091(9) 0.0086(5) 0.2454(5) 0.079(9) 0.13(1) 0.093(9) 0.004(8) -0.002(7) 0.040(8) C(36) 8c 0.474(1) 0.0181(5) 0.3084(4) 0.21(2) 0.19(1) 0.064(8) -0.01(1) 0.017(9) 0.029(7) C(37) 8c 0.6168(9) -0.0273(6) 0.2433(5) 0.13(1) 0.23(2) 0.19(1) 0.07(1) 0.02(1) 0.10(1) C(38) 8c 0.4250(9) -0.0320(4) 0.2171(4) 0.16(1) 0.096(8) 0.13(1) -0.011(8) 0.001(9) 0.045(7)
Acknowledgments. We like to express our thanks to the Fonds der Chemischen Industrie, the Volkswagen Stiftung and Hewlett Packard GmbH for their generous support.
References
1. Rieker, Α.: ElektronenUbergänge zwischen Resonanzsystemen/Ein Beitrag zur Kenntnis der Aroxyle. Dissertation, University of Tübingen, Germany 1961.
2. Ley, К.; Müller, В.; Mayer, R.; Scheffler, К.: Dimerisierende Dehydrie- rung von Phenolen mittels 2,4,6-Tri-/írt-butyl-phenoxyls-( 1 ). Chem. Ber.
91(1958)2670-2681.
3. Müller, Е.; Rieker, Α.; Schick, Α.: Dehydrierungs-Additions-Reaktionen mit Aroxylen, 1. Neue radikalisch verlaufende Synthesen von Arylalkyle- them, Arylestem, Chinolethem und /J-Hydroxydiphenylethem. Liebigs Ann. Chem. 673 ( 1964) 40-59.
4. Müller, E.: Rieker, Α.; Mayer, R.; Scheffler, K.: Dehydrierung sterisch behinderter Phenole unter Bildung "einfacher" dissoziabler Chinolether.
Liebigs Ann. Chem. 645 (1961) 36-52.
C 3 0 H 4 0 N 2 O 2 S 2
187
5. Spek, Α. L.: PLATON, an integrated tool for the analysis of the results of a single crystal structure determination. Acta Crystallogr. A46 (1990) C34.
6. Spek, A. L.: Program PLATON Version 310596 for geometric calculati- ons. University of Utrecht, The Netherlands 1996.
7. Boyens, J. C. Α.: The conformation of six-membered rings. J. Cryst. Mol.
Struct. 8(1978)317-321.
8. Henes. G.; Rieker. Α.; Neumayer, M.; Hiller, W.: 1.4-Addition of lithium organyls to para-quinols / Structure determination of 2,6-di-ferf-butyl-4- hydroxy-4.5-diphenylcyclohex-2-en-l-one. Z. Naturforsch. S i b (19%) 381-387.
9. Altomare, G.; Cascarano, G.; Giaccovazzo, С.; Guagliardi, Α.; Burla, M.
е . ; Polidori, G.: SIR92 - a program for automatic solution of crystal structures by direct methodes. J. Appi. Crystallogr. 27 (1994) 435.
10. Kopf, J.; Rübcke, H.-Chr.: Program CADSHEL for data reduction. Uni- versity of Hamburg, Germany 1993.
11. NONIUS B.V.. four-ciicle diffractometer CAD4, Delft, The Netheriands.
12. Sheldrick, G. M.: Program SHELXL-93, a program for the refmement of crystal structures. University of Göttingen, Germany 1993.
13. AXS Analytical X-Ray Systems GmbH: Program package SHELXTL, Rei. 5.03. Karlsruhe, Germany 1995.