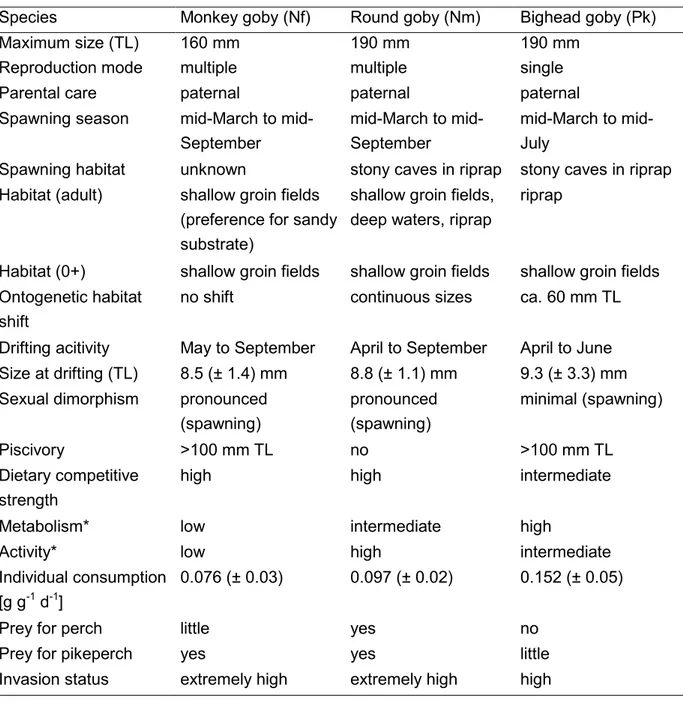
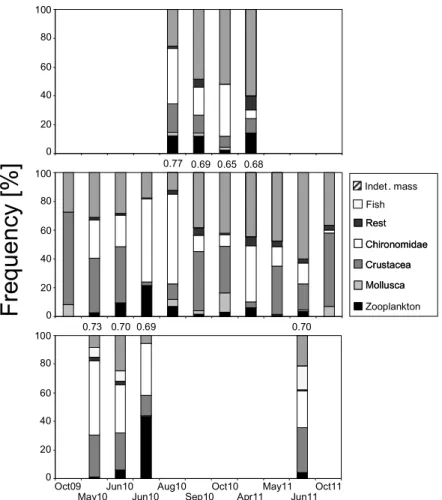
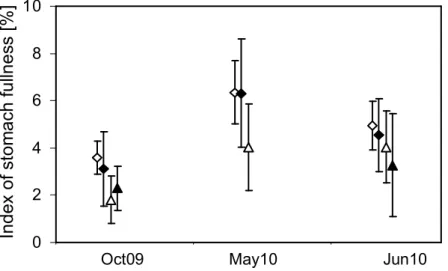
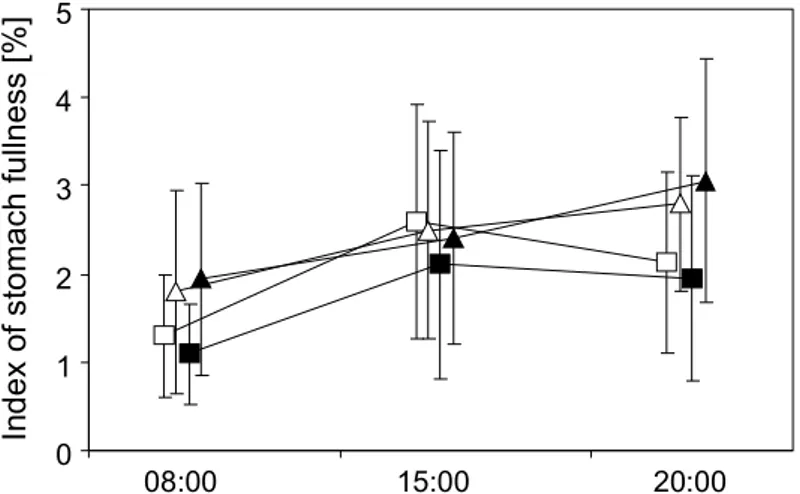
The ecological niche of invasive gobies at the Lower Rhine in intra- and interspecific competitive and predatory interactions
Volltext
Abbildung



ÄHNLICHE DOKUMENTE
Eurasian perch Perca fluviatilis (henceforth: perch) is a model species in freshwater fish ecology used extensively in field stud- ies and in controlled aquarium and mesocosm
shoals as was shown in a previous genetic study (Gerlach et al., 2001). Group pref- erence is based on olfactory preference for related individuals and if it lasted during
Though there is only one specimen of the OTU 7, but considerable differences are recognized between OTU 5 and OTU 7 in the high numbers of upper labials (9.50 vs. robustus) in
We used perch (Perca fluviatilis) as a visually orientated, and ruffe (Gymnocephalus cernuus) as a mechano-sensory oriented predator and tested their growth rates and behaviour
Overall, inoculation with mycorrhi zal fungi tended to reduce total plant biomass (marginally significant mycorrhiza effect in Table 2), both for invasive and non invasive alien
The higher growth rates of ruffe found by Schleuter and Eckmann (2006) in experi- ments with groups of four as compared to those of F IGURE 6.—Mean 6 SD hourly based routine
The total abundance of egg ribbons did not differ significantly between the two sites, but the preferred spawning depth was deeper at the wave exposed site (5 m) compared
Hence, inter- locus gene-conversion can be particularly effective in increas- ing the allelic repertoire, and might be important in species where a high number of MHII 13 loci