Synthesis of mannose extended N- and O-glycopeptides for antibody generation and studies of protein-carbohydrate interactions
Volltext
Abbildung

ÄHNLICHE DOKUMENTE
Key words: Potassium, Hydroxylamine, Aggregate, Bond Cleavage,
Two unnatural α -amino acid esters were prepared in good yields via phase transfer catalyzed Michael addition of ethyl N-acetylaminocyanoacetate to chalcone and benzalketone.. For
Two unnatural α -amino acid esters were prepared in good yields via phase transfer catalyzed Michael addition of ethyl N-acetylaminocyanoacetate to chalcone and benzalketone.. For
~ 4.32, corresponding to a randomly generated se- quence (1/20 probability of finding any one of the 20 amino acids at any given site). This level of complexity is never realized
[r]
The second group II in which a guanidine is incorporated instead of the second bis(Zn(II)-cyclen) triazine complex 16 should have a high affinity to peptides containing
In the heart conditional Hccs KO model, parts of the Hccs gene are specifically knocked out in cardiomyocytes from 7.5 dpc onwards applying Cre/locus of X-over P1 (loxP).. In
The criteria used to describe the influence of the investigated amino acids on the nucleation of cal- cium carbonate are; (1) the slope of the linear increase during the
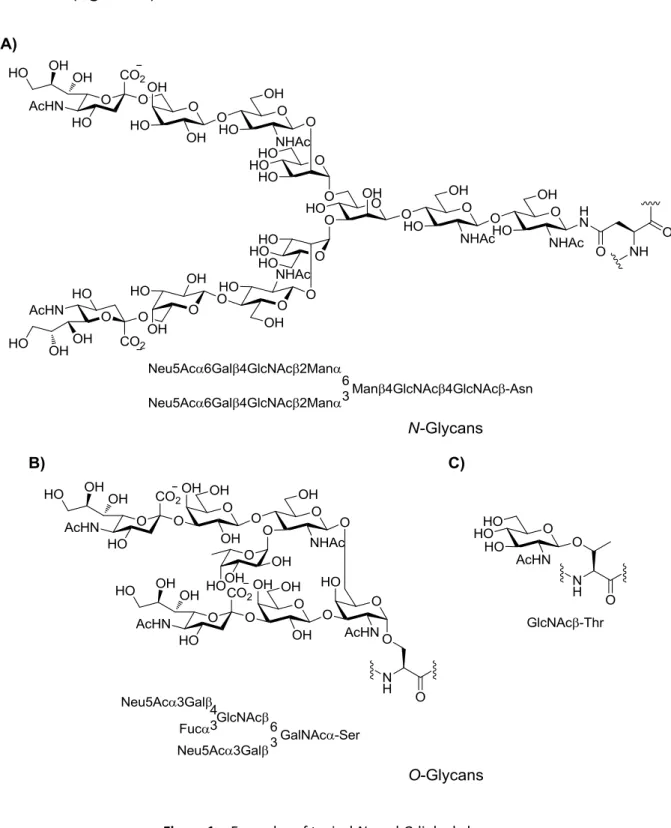
![Figure 9. Homogeneous native N-glycan structures. [123]](https://thumb-eu.123doks.com/thumbv2/1library_info/3632523.1502276/50.892.220.672.418.805/figure-homogeneous-native-n-glycan-structures.webp)