DISS. ETH NO. 27414
Computational Modeling and Simulation of the Shoulder for Functional Analysis of
Orthopedic Surgery Outcomes
A dissertation submitted to attain the degree of
DOCTOR OF SCIENCES of ETH ZURICH (Dr. sc. ETH Zürich)
presented by
Fabien Péan
Master of Science, Technische Universität München Ingénieur diplômé de l’École Centrale de Lille
born on 10. July 1990 citizen of France
accepted on the recommendation of Prof. Dr. Orçun Göksel, examiner Prof. Dr. Yohan Payan, co-examiner
Dr. Philippe Favre, co-examiner
2021
Dedicated to my beloved Aynur and my sweet niece Lucía
Abstract
The shoulder offers the greatest range-of-motion in the whole human body and plays a key role in enabling the execution of common daily tasks vital for remaining independent, which is only possible thanks to its unique structure. Indeed, the shoulder consists of loosely connected bones maintained in their desired positions by many muscles, which play a vital role in moving the arm and transferring the load from the upper limb to the trunk. However, it means that the various observable properties are the result of a complex set of neurological processes, which coordinates the many muscles for actuating the upper limb. Active motion, as the result of conscious decision, is a key aspect of understanding pathology and their potential solution. Unfortunately, it is this particular aspect that is difficult to study for practical and ethical reasons. Alternative to clinical and ex-vivo studies falling under the aforementioned concerns, numerical models of the musculoskeletal system offer a great opportunity for an ethical, reproducible, and flexible means to analyze in-depth the human movement and its intrinsic properties. However, several outstanding challenges in modeling have long delayed the application of such models in concrete clinical and biomechanical scenarios. Validation of how to be certain that a model can provide meaningful results has also been a long-standing question in the field.
In this thesis, musculoskeletal models of the shoulder are developed with the aim of deepening the understanding of orthopedic conditions their surgical treatment options.
Two musculoskeletal models are presented, respectively, using volumetric and surface- based elements: The former is a fully volumetric and continuum-based musculoskeletal model of the shoulder joint and girdle. Its simulation is faster than usual volumetric models owing to a simpler geometrical representation of the muscles, while retaining a similar degree of fidelity to the anatomical knowledge. Muscles of the shoulder girdle are all integrated with a free-hanging scapula, whose muscles are controlled by a position-tracking controller. Such a comprehensive model allows for analyzing scapular dysfunction as well as glenohumeral pathologies and their impact on the arm movement.
The latter musculoskeletal model presents an alternative to earlier modeling paradigms, wherein muscle segments are represented as thin-shell surface elements instead of the more common lines or volumetric elements. The proposed representation combines the advantages of both alternative representations. Indeed, surface-based muscle segments are easy to generate and fast to simulate, allows for spatial variation of biomechanical parameters thanks to the texture-like visualization of such model, and do not require manual intervention to enforce a muscle to pass along anatomically known positions.
The proposed surface-based model, being superior, is then used in the context of two clinical conditions and their surgical corrections thereof. For these two applications, the model is validated extensively against available in-vivo measurements. Shoulder v
vi
arthroplasty is a common surgical procedures and reverse shoulder arthroplasty involves the replacement of the humerus head by a socket-shaped prosthesis, and the glenoid by a ball-shaped prosthesis. Reverse arthroplasty is traditionally applied when the rotator cuff is severely deficient, however it is getting more common even when the rotator cuff is still functional, owing to the superior stability properties of this type of prosthesis. Therefore, it is vital to characterize the impact of the surgery with a near functional rotator cuff, especially considering the forces within the glenohumeral joint, which are relevant for assessing the lifetime of the prosthesis. In simulations, increased compression forces within the joint have been found, which may indicate an increased rate of wear of the prosthesis. As a result, the procedure may increase the rate of revision surgeries, which is a concern when applied on a younger population. If the rotator cuff is nearly intact, consisting of only one or two irreparable torn tendons, a surgical option of muscle transfer may be preferred. It involves moving the attachment of an intact muscle onto the area of a ruptured muscle in order for the transferred muscle to replace some of its lost function. The pectoralis major is the main muscle considered for replacing an irreparable subscapularis. However, very few biomechanical studies are available to understand this procedure and its impact on active motion during activities of daily living. Subscapularis tears are found to require a compensatory activation of the supraspinatus and is accompanied by a reduced co-contraction of the infraspinatus, both of which can be partially recovered after pectoralis major transfer. However, while the subscapularis and pectoralis major fulfill some similar functions, their actions is generally asynchronous and their synergistic action highly motion-dependent, both of those can change significantly after the transfer. Such differences may explain the difficulty of some patients in adapting their psycho-motor patterns during the rehabilitation period, whereby biofeedback exercise may be recommended. In addition, following a transfer, other muscles need to compensate for the absence of the transferred muscle, in turn, which hints at the complex, inter-connected relations between the shoulder muscles, motivating the need for empirical studies and functional simulations to test different scenarios.
Résumé
L’épaule offre la plus grande amplitude de mouvement de tout le corps humain et joue un rôle clé en permettant l’exécution des tâches quotidiennes courantes vitales pour rester indépendant, ce qui est possible grâce à sa structure unique. En effet, l’épaule est constituée d’os faiblement connectés maintenus en positions par de nombreux muscles, qui jouent un rôle essentiel dans le déplacement du bras et le transfert de la charge du membre supérieur au tronc. Cependant, cela signifie que les différentes propriétés observables sont le résultat d’un ensemble complexe de processus neurologiques, qui coordonne les nombreux muscles pour actionner le membre supérieur. Le mouvement actif, résultat d’une décision consciente, est un aspect clé de la compréhension de la pathologie et de la solution envisagée. Malheureusement, c’est cet aspect particulier qui est difficile à étudier pour des raisons pratiques et éthiques. En alternative aux études cliniques et ex-vivo relevant des préoccupations susmentionnées, les modèles numériques du système musculo-squelettique humain offrent une opportunité pour un moyen éthique, reproductible et flexible d’analyser en profondeur le mouvement et ses propriétés intrinsèques. Cependant, plusieurs défis majeurs dans la modélisation ont longtemps retardé l’application de tels modèles dans des scénarios cliniques et biomécaniques concrets. Obtenir l’assurance qu’un modèle peut fournir des résultats significatifs est également un souci récurrent dans le domaine.
Dans cette thèse, des modèles musculo-squelettiques de l’épaule sont développés dans le but d’approfondir la compréhension des problèmes orthopédiques et de leurs traitement chirurgical. Deux modèles musculo-squelettiques sont présentés, le premier utilisant des éléments volumétriques et le second des éléments surfaciques : Le premier modèle est un modèle musculo-squelettique entièrement volumétrique de l’articulation de l’épaule et de la ceinture scapulaire, basé sur la mécanique des milieux continus. Le temps de calcul est plus rapide que les modèles volumétriques habituels grâce à une simplification géométrique des muscles, tout en conservant un degré similaire de fidélité aux connais- sances anatomiques. Les mouvements de l’omoplate sont libres et tous les muscles de la ceinture scapulaire sont présents, ceux-ci étant activés par un contrôleur de suivi de position. Un tel modèle permet d’analyser le dysfonctionnement scapulaire ainsi que les pathologies glénohumérales et leur impact sur le mouvement du bras. Le second modèle musculo-squelettique présente une alternative aux paradigmes de modélisation antérieurs, dans lesquels les muscles sont représentés par des éléments surfaciques au lieu des éléments linéaires ou volumétriques plus courants. La représentation proposée combine les avantages des deux approches antérieures. En effet, les faisceaux musculaires représentés en surface sont faciles à générer et rapides à simuler, permettent une variation spatiale des paramètres biomécaniques grâce à la visualisation en texture d’un tel modèle, et ne nécessitent pas d’intervention manuelle pour forcer un muscle à passer le long de positions anatomiquement connues.
vii
viii
Le modèle en éléments surfaciques proposé, étant supérieur, est utilisé dans le cadre de deux pathologies et de leurs corrections chirurgicales. Pour ces deux applications, le modèle est validé à plusieurs reprises par rapport aux mesures in-vivo disponibles.
L’arthroplastie de l’épaule est une intervention chirurgicale courante et l’arthroplastie de l’épaule inversée implique le remplacement de la tête de humérus par une prothèse en forme de réceptacle et de la cavité glénoïde par une prothèse en forme de boule.
L’arthroplastie inversée est habituellement mise en place lorsque la coiffe du rotateur est sévèrement déficiente, mais elle devient plus couramment appliquée même lorsque celle-ci est encore intacte, en raison de la stabilité supérieure offerte par ce type de prothèse. Il est donc essentiel de caractériser l’impact de la procédure chirurgicale lorsque la coiffe du rotateur est encore fonctionnelle, en particulier vis-à-vis des forces opérant au sein de l’articulation gléno-humérale, qui sont cruciales pour évaluer la durée de vie de la prothèse. Dans les simulations effectuées, des forces de compression accrues dans l’articulation ont été observées, ce qui peut indiquer une augmentation du taux d’usure de la prothèse. En conséquence, la procédure peut augmenter le taux de chirurgies de révision, ce qui est une préoccupation lorsqu’elle est effectuée sur une population relativement jeune. Si la coiffe du rotateur est presque intacte, constituée d’un ou de deux tendons déchirés et irréparables, un transfert musculaire peut cependant être préféré. Il s’agit de déplacer la terminaison d’un muscle intact sur la zone d’un tendon rompu afin que le muscle transféré remplace une partie des fonctions du muscle rompu. Le grand pectoral est le muscle principal considéré pour remplacer un sous-scapulaire irréparable.
Cependant, très peu d’études biomécaniques sont disponibles pour comprendre cette procédure et son impact sur le mouvement actif lors des activités de la vie quotidienne.
Les déchirures du sous-scapulaires nécessitent une activation compensatrice du supra- épineux et s’accompagnent d’une co-contraction réduite de l’infra-épineux, qui peuvent être partiellement rétablis après un transfert du grand pectoral. Cependant, bien que le sous-scapulaire et le grand pectoral remplissent certaines fonctions similaires, leurs actions sont généralement asynchrones et leur action synergique varie fortement en fonction du mouvement étudié, ces deux facteurs pouvant changer considérablement après le transfert. Ces différences peuvent expliquer la difficulté de certains patients à adapter leurs régimes psycho-moteurs pendant la période de rééducation, moyennant quoi des exercices de rétroaction biologique peuvent être recommandés. De plus, à la suite d’un transfert, d’autres muscles doivent compenser à leur tour l’absence du muscle transféré, ce qui laisse entrevoir les relations complexes et interdépendantes entre les muscles de l’épaule, motivant le besoin d’études empiriques et de simulations fonctionnelles pour tester différents scénarios.
Acknowledgements
The research developed throughout this thesis was supported by the highly specialized medicine grant (HSM2) of the Canton of Zurich and the Surgent project of the University Medicine Zurich.
First and foremost, I would like to thank myDoktorvaterProf. Dr. Orçun Göksel, for taking me under his wing and without whom this adventure would have not been possible. I am grateful for the time he invested in me and the guidance he provided on many aspects of my thesis.
I would like to extend my gratitude to the rest of my thesis committee, Dr. Philippe Favre and Prof. Dr. Yohan Payan, for devoting their time reviewing my dissertation. I want to thank all my collaborators, that through these years, whether by fostering discussions or their interest, allowed me to see the value and be confident about my work, thank you Dr.
Philippe Favre, Prof. Dr. Philipp Fürnstahl, Dr. Christine Tanner, and Prof. em. Christian Gerber. Thanks to Dr. John Lloyd, Dr. Antoine Perrier, Prof. Dr. Yohan Payan, and Dr. Soo Kim, whom I had the pleasure to meet during conferences, for the in-depth discussions and for introducing me to the community.
My appreciation also goes to many in Balgrist, CVL, and CAiM, with whom I had the pleasure to interact with throughout these years. Many thanks to Dr. Fabio Carrillo, Dr. Hayko Riemenschneider, Dr. Thomas Probst, Dr. Danda Paudel, Dr. Ajad Chhatkuli, Kerem Can Tezcan, Martin Hahner, Dr. Kenneth Vanhoey, Dr. Christian Baumgartner, Prof. Dr. Ender Konuko ˘glu, Dr. Sergi Caelles, Christos Sakaridis, Cadu Porto de Oliveira, Anna Volokitin, Christina Krueger, Dr. Till Kroeger, Dr. Maxim Makhinya, Dr. Oliver Mattausch, Lin Zhang, Dr. Richard Rau, Domenico Ferrara, Dr. Baran Gözcü, Dr. Tiziano Portenier, Dieter Schweizer, Dr. Antonio Foncubierta and countless others.
Particularly, I would like to express my gratitude to some amazing teammates. First, to the two other members of the prime ETF-C111 trio: Dr. Firat Özdemir and Dr. Ece Özkan-Elsen. The best officemates I could hope for, with whom I had some much fun, inside and beyond the lab. Our lively discussions helped me go through many things, professionally and personally. And secondly, to some members of the unofficial CAiM kicker team: Matija Ciganovic, Rastislav Starkov, Alvaro Gomariz Carrillo, and Baskhara Rao Chintada, for all the entertaining conversations during coffee breaks and elsewhere.
Thanks to all my friends I met throughout my life, whether in Sarthe, Lille, Munich, or Zurich, for their support and with whom I always enjoy to crack some jokes with. Special mention to Kevin, for our lengthy evening chat sharing respective life experience, time online, and raclette irl, which greatly lifted up my spirits.
ix
x
I am grateful to my parents Martine and Jean, who took care of me for so long, from my early tantrums to my teenage whims, and provided the resources for my post-secondary education, without which I would not have been able to start this doctorate. Also to my brother Arnaud, his wife Barbara and my niece Lucía, who was the daily entertainment for the last years of my doctorate.
Last but not least, many thanks to my beloved Aynur, who helped me through my ups and downs with kindness, the anchor to my wavy world. Thank you Nunur.
Contents
1 Introduction 1
1.1 Anatomy of the Shoulder . . . .3
1.2 Modeling of the Shoulder Muscles . . . .5
1.3 Modeling of Shoulder Articulation . . . .6
1.4 Shoulder Arthroplasty. . . .7
1.5 Muscle Transfer . . . .8
1.6 Thesis Goals . . . .9
1.7 Thesis Outline and Contributions . . . 11
2 A Comprehensive and Volumetric Musculoskeletal Model for the Dynamic Simulation of the Shoulder Function 13 2.1 Introduction. . . 13
2.2 Methods . . . 16
2.2.1 Geometric model . . . 16
2.2.2 Material properties . . . 19
2.2.3 Validation . . . 20
2.2.4 Simulations . . . 20
2.3 Experimental Evaluation and Results . . . 21
2.3.1 Muscle moment arms comparisons . . . 21
2.3.2 Pathological scenario: scapular winging . . . 22
2.4 Discussion. . . 23
2.5 Conclusions . . . 27
3 Surface-based Modeling of Muscles: Functional Simulation of the Shoulder29 3.1 Introduction. . . 30
3.2 Methods . . . 32
3.2.1 Shoulder Muscles . . . 32
3.2.2 Surface Models . . . 33
3.2.3 Automatic Generation of Muscle Surface Models . . . 35
3.2.4 Simulation . . . 36
3.2.5 Control of the Musculoskeletal Model . . . 37
3.2.6 Implementation . . . 38
3.3 Simulations and Results. . . 40
3.3.1 Evaluation of our Model Generation for plausible Bone Wrapping 40 3.3.2 Demonstration of Spatially-varying Muscle Parameters . . . 41
3.3.3 Performance with respect to volumetric approach . . . 41
3.3.4 Activities of Daily Living . . . 43 xi
xii c o n t e n t s
3.3.5 Pathology Simulation . . . 43
3.4 Discussion. . . 46
3.5 Conclusions . . . 49
4 Influence of Rotator Cuff Integrity on Loading and Kinematics: Before and After Reverse Shoulder Arthroplasty 51 4.1 Introduction. . . 51
4.2 Methods . . . 52
4.2.1 Musculoskeletal Model of the Shoulder . . . 52
4.2.2 Subject-specific Modeling . . . 53
4.2.3 Simulation of Rotator Cuff Tears and Reverse Shoulder Arthroplasty53 4.2.4 Model Validation . . . 54
4.2.5 Analysis . . . 55
4.3 Results . . . 55
4.3.1 Validation . . . 55
4.3.2 Range-of-motion . . . 55
4.3.3 Flexion with Tears of the Rotator Cuff . . . 55
4.3.4 Flexion with Reverse Shoulder Arthroplasty . . . 57
4.4 Discussion. . . 58
4.5 Conclusions . . . 60
5 Computational Analysis of Subscapularis Tears and Pectoralis Major Trans- fers on Muscular Activity 61 5.1 Introduction. . . 62
5.2 Methods . . . 63
5.2.1 Musculoskeletal Model of the Shoulder . . . 63
5.2.2 Subject-specific Modeling . . . 64
5.2.3 Electromyography (EMG) . . . 64
5.2.4 Comparison to in-vivo Joint Reaction Forces . . . 64
5.2.5 Simulation of Pectoralis Major Transfer . . . 64
5.2.6 Analysis of the Tear and the Muscle Transfers . . . 65
5.3 Results . . . 66
5.3.1 Model Validation . . . 66
5.3.2 Muscle Activity . . . 67
5.4 Discussion. . . 69
5.5 Conclusions . . . 72
6 Summary and Perspectives 75 6.1 Summary . . . 75
6.2 Future Work . . . 77 A Subject-specific transformation of tracked motion to model coordinates 93
List of Figures
1.1 Skeletal anatomy of the shoulder [Betts et al.,2013]. . . .3 1.2 Muscles of the shoulder, adapted from [Betts et al.,2013]. . . .4 1.3 Various approaches to musculoskeletal modeling. From left to right, the
illustrations are from [Chadwick et al.,2014], [Webb et al.,2014], and [Teran et al.,2005]. . . .6 1.4 Total Shoulder Arthroplasty (left) and Reverse Total Shoulder Arthroplasty
(right). Used with permission of Mayo Foundation for Medical Education and Research, all rights reserved. . . .8 1.5 Intact anatomy (left), pectoralis major transfer of the clavicular part (middle)
and sternal part (right). Illustrations of the transfer from [Talbot et al.,2013].10 2.1 Connectivity diagram of the musculoskeletal system of the shoulder. . . . 14 2.2 Procedure used to model a muscle (here the deltoid, acromial part). From left
to right: the original surface mesh, its model as a smooth CAD object, which is then meshed, and mapped with the fiber orientations that we generate based on the gradients of a diffusion (Laplace) equation between the muscle origin and insertion points. . . 18 2.3 Front, side, and back view of the proposed model . . . 18 2.4 (a) Dynamic Arm Simulator of [Chadwick et al., 2014] showing that an
ellipsoid may not be sufficient to geometrically approximate the ribcage.
(b-d) Front, side, and back views of our ribcage mesh triangulated from a NURBS surface fitted to the given ribcage anatomy. . . 19 2.5 Moment arms (cm) from our simulation of shoulder abduction in frontal
plane (bold lines) overlaid on [Webb et al.,2014] for a thoracohumeral angle ranging from 0 to 90°. The resulting moment arms from our simulations are scaled by23 to account for the transformation from a glenohumeral angle to a thoracohumeral one. For the deltoid, colours differentiate the anterior part (red), middle part (green), and posterior part (blue). . . 23 2.6 Moment arms (cm) from our simulation of shoulder axial rotation (bold lines)
overlaid on [Webb et al.,2014]. For the deltoid, colours differentiate the anterior part (red), middle part (green), and posterior part (blue). . . 24 2.7 Moment arms (cm) of shoulder abduction in scapular plane. Comparison
from our simulation (solid lines) with ex-vivo data from [Ackland et al., 2008] (dotted lines). The colour coding reads: red, green, blue for superior or anterior, middle, inferior or posterior, respectively. Black lines refer to the Teres Minor. . . 25
xiii
xiv l i s t o f f i g u r e s
2.8 The end positions of the scapula after pure protraction simulations are shown from the top (top) and back (bottom) views. The blue and red scapula refer to the end positions for simulations with a normal and paralyzed serratus anterior, respectively. The green and purple guides highlight the positional difference between the healthy and pathological cases. . . 26 2.9 Muscle activation during the simulation of serratus anterior paralysis during
forward humeral head motion (middle) in comparison to the physiological case (left). . . 27 3.1 Shear test for a clamped thin structure. Linear elements are shown in red,
green and cyan, with respectively 1, 2 and 20 elements over the thickness.
The magenta mesh is a B-Spline volume with p=2 and 1 element over the thickness. Magenta and cyan meshes both converge toward the same solution.
The higher-order mesh require far fewer elements compared to the linear ones. . . 32 3.2 Illustration of the automatic surface model generation process for a sample
muscle (infraspinatus). The steps are:(a)resample origin (blue) and inser- tion (green) curves,(b)create intermediate points (red),(c)generate B-spline surface,(d)apply force along surface normals,(e)remove forces and enable collisions with bones. . . 35 3.3 Illustration of our kinematics chain. Landmarks for the motion tracking are
displayed as green points. Acromioclavicular ligaments are shown in blue and cyan, while coracoclavicular ligaments are shown in red and magenta. 39 3.4 Automatic generation of models for muscle models, with the proposed
surface models (top row) and with a state-of-the-art line-segment approach (bottom row). Posterior(a)and anterior(b)views are shown; as well as infraspinatus(c)and deltoid(d)in isolation, where nonphysiological line- segment wrappings are colored in red. With line-segments, the fibers appear spread or lumped nonphysiologically, leading potentially to false moment arms. . . 40 3.5 Simple synthetic examples of surface models demonstrating muscle deforma-
tion after full activation(b)along illustrated fibers, where local paralysis is simulated as activation loss at the darker regions shown at rest(a). Muscles are attached between the gray boxes, which are fixed in space. Result- ing forces and moments shown indicate an expected strength loss and an asymmetric pull. . . 42 3.6 Examples of spatial parametrization of surface models using texture maps:
in(a), a grayscale texture defines muscle and tendon ratio, exemplifying a gradual transition with a complex spatial arrangement. Red indicates muscle and cyan tendon, while length show the proportion of each. In(b), the orientation of muscle fibers are defined as an RGB texture, with color channels used as a vector field. . . 43 3.7 Simulation time using surface compared to volume model. The surface of the
model is shown in cyan, while the nodes are in green and the rigid sphere colliding with the soft model is in magenta. . . 44 3.8 Snapshots from simulated motions of ADL from antero-lateral (top) and
postero-lateral (bottom) views. . . 45
l i s t o f f i g u r e s xv
3.9 In(a)left, activations of all muscles over time for the physiological case are shown. In(a)right, the difference between pathological and physiological activations are shown for a subset of muscles, italicized in the legend, for those having a Root Mean Square difference above 5%. In(b)left, norm of force acting on the insertion for of all muscles over time for the physiolog- ical case are shown. In(b)right, the difference between pathological and physiological forces are shown for the same subset of muscles as in(a)right.47 3.10 In(a)left, joint reaction forces within the glenohumeral joint are given for both
physiological(green) and pathological(red) cases. Compressive force is de- noted as solid line, while shear force is dashed. In(a)right, the deviation an- gle from pure compressive force is given asθ=arctan(||fshear||/||fcompr||). In(b)left, distance between the physiological and pathological tracked land- marks are shown. In(b)right, the orientation of the scapula over time is given for both physiological (green) and pathological (red) cases. For visualization purposes, data were downsampled by decimating with a factor of 10. . . 48 4.1 The effect of reverse prosthesis (green) modeled in our simulation by shifting
the center of rotation (dots) of the glenohumeral joint [Boileau et al.,2005], with red showing the intact case. Outlines of the humerus are shown as solid lines. . . 54 4.2 Comparison of peak JRF during anterior flexion up to 90° for slow forward
flexion motion between the model and in-vivo measurements [Bergmann et al.,2011]. . . 56 4.3 JRF during anterior flexion for our simulation, in-vivo measurements of
subjects S1 and S2 from Orthoload (OL) [Bergmann] and simulated subjects with DSEM [Nikooyan et al.,2010]. . . 56 4.4 Force magnitude in the glenohumeral joint during anterior flexion (a,d),
detailed into its compressive (b,e) and shear (c,f) components, for different rotator cuff tear conditions (a,b,c) and with reverse implant (d,e,f). The range when JRF points outside the glenoid is shown as a horizontal bar in the JRF graph with the color corresponding to muscle condition in (a). . . 57 4.5 Upward rotation of the scapula for the different rotator cuff tears (a) and
reverse arthroplasty (b) conditions. The intact anatomy baseline is shown as a solid black line. . . 58 5.1 Intact PMA(a)simulated transfer of(b)clavicular part,(c)sternal part, and
(d)sternoclavicular part. Pectoralis major clavicular, sternal, abdominal parts are shown in yellow, green, blue, respectively. . . 65 5.2 Joint Reaction Force magnitude for the combing motion. In-vivo measure-
ments are shown in black and current modeling results in blue. All curves are time-normalized to the range [0,1]. . . 67 5.3 Computed activation of the SSP for each simulated ADL for all simulated
cases. . . 69 5.4 Computed activation of the ISP for each simulated ADL for all cases. Shaded
areas correspond to time windows where activation of the SSC in the intact case is below a threshold of 1%, indicating the absence of co-contraction of SSC and ISP. . . 70
xvi l i s t o f f i g u r e s
5.5 Computed activation of the DAN for each simulated ADL for the intact, torn SSC, and transfers involving PMAC cases. Activation of the intact PMAC is shown for comparison (black solid line). . . 71 5.6 Computed activation of the PMAC (solid lines) and PMAS (dashed lines)
for each simulated ADL for the intact and torn SSC cases, and transfer cases involving the respective muscle segments transfer. Activation of the intact SSC is shown for comparison (black solid line). . . 73
List of Tables
2.1 Summary of anatomical structures included in our model . . . 17 3.1 Shape categorization of muscles based on their origin and insertion sites . 33 3.2 Time [ms] of the simulation described inFigure 3.7 . . . 41 3.3 RMSE of landmarks on the humerus (EM, EL) and on the scapula (AI, AA,
PC, TS) after simulating four ADL with given duration. . . 46 4.1 Range-of-motion during anterior flexion with (+rev) and without reverse
prosthesis. . . 55 4.2 Mean difference in JRF with respect to intact case during anterior flexion
with (+rev) and without prosthesis. . . 58 5.1 List of muscle segments in the model with their abbreviations. . . 63 5.2 Distance, mean±SD (max), between landmarks located at the humeral tip,
EM and EL, and their respective target positions from motion capture data.66 5.3 Distance, mean±SD (max), between the simulated intact motion and the
simulated clinical conditions of the humerus landmarks EM and EL. . . . 66 5.4 For the intact scenario, Spearman correlation coefficientsxcorrbetween simu-
lated activations and the processed EMG signals from the reference dataset.
Cells are color coded from -1 in red to 1 in green. Muscle abbreviations are listed in Table5.1. . . 67 5.5 Mean difference of SSP activation after transfers with respect to its activation
in the torn SSC case, i.e.∑iN 1
N(acasessp(ti)−a-sscssp(ti)), shown in the format mean±SD. . . 68 5.6 Mean difference of ISP activation after transfers with respect to its activation
in the torn SSC case, i.e.∑iN 1
N(acaseisp (ti)−a-sscisp(ti)), shown in the format mean±SD. . . 68 5.7 Percentage of the time during which the PMAC and PMAS are active (≥1%)
at the same time as SSC was in the intact case. Cells are color coded from 0 in red to 100 in green. . . 72 5.8 Spearman correlation coefficients between the activation signal of SSC in the
intact case and the activation signals of PMAC and PMAS in intact case and after both transfer. Cells are color coded from -1 in red to 1 in green. Empty cells indicate no correlation (ρ=NaN), as PMAS shows no activation in the intact case. . . 72
xvii
xviii l i s t o f ta b l e s
5.9 Spearman correlation coefficients between activation signals in the intact case and with torn SSC and after both transfer. Cells are color coded from -1 in red to 1 in green. Empty cells indicate no correlation (ρ=NaN), as PMAS shows no activation in the intact case. . . 74 5.10 Stability of the glenohumeral joint, indicated as the angle [°], mean±SD (max),
between the JRF and the pure compression direction. . . 74
1
Introduction
The human body is a complex machinery of interleaved systems. Among them, the musculoskeletal system, with its 206 bones, over 650 named skeletal muscles and various connective tissue, constitutes over half of the body mass. It is the system responsible for achieving motion and carrying loads, which is a vital function for performing daily activities autonomously. Like other parts of the human body, the musculoskeletal system is also susceptible to impairment and dysfunction. Musculoskeletal impairments generally induce pain, limited mobility and reduced strength, and occurs more frequently with age.
These do not generally lead to life threatening conditions, such as what cancer might do, nevertheless, given their negative consequences on everyday life and the aging world population, musculoskeletal problems are a primary concern in healthcare worldwide.
In 2016 in the USA, orthopedic surgeries made up almost a quarter of all operating room procedures performed during hospital stays [Weiss et al.,2014]. The occurrence of revisions and re-intervention surgeries, which may be indicated when the musculoskeletal problem is not remedied or for wear of a prosthesis or failure to treat the problem, is noticeably high in orthopedics. For example, in 2016 in Switzerland, revisions accounts for 10% and 12%, respectively, of knee and hip arthroplasties [Beck et al.,2019].
Advances in medical imaging techniques allow to observe inside the body with an ever in- creasing detail and contrast modalities. Noticeably MRI is a safe option to obtain detailed images of the musculoskeletal structures. However, medical images often provide only static snapshot of the body, although kinematics is particularly important to understand motion. Some imaging protocols are promising in recording time evolution of the inner body, such as dynamic MRI, currently at the cost of a much lower resolution [Pierrart et al.,2014; Tempelaere et al.,2016]. Imaging protocols only allow to observe the current geometric state of the internal structures, whereas a broad range of musculoskeletal pathologies relate to the evolution of forces and neuromotor functions within the articu- lation and muscles. Other devices, such as force plates or dynamometers, enable only an indirect force measurement. Unfortunately, recording of internal forces cannot be performed without invasive procedures, whether in clinical or research conditions. Due to the inability for direct observation and repeatable experimentation of different scenarios on the same subject, clinical research in orthopedics follows atrial and errorapproach.
Indeed, following anatomical and ex-vivo biomechanical studies, new surgical proce- dures are directly tested on consenting patients following ethical regulations, to reach conclusions based on case-based or statistical outcome observations. Outcomes are highly 1
2 1 i n t r o d u c t i o n
variable owing to inter-subject variability, small population samples, and often varying populations, norms and practices across practicing centres requiring numerous attempts before drawing reliable conclusions and general guidelines. Thorough understanding of the musculoskeletal system is only achievable by compounding in-vivo, dynamical and neuromuscular measurements, all of which are hardly attainable in clinical or traditional biomechanical research.
Computational models of the musculoskeletal system is an ideal alternative to address the shortcomings with anatomical, clinical and traditional biomechanical studies. Muscu- loskeletal models enable to mimic in-vivo conditions and can provide many unobservable values of interest of the musculoskeletal system. Such models are currently constructed following two main approaches. The first one relies on multi-body dynamics, where bones are rigid bodies, articulations are kinematic joints and muscles are represented as polylines wrapping over the bones. Such approximation is usually used to model large extents of the musculoskeletal system. Models based on this technique are capable of estimating muscles activation, as well as forces within muscles and articulations, during dynamic movements. A second approach relies on continuum mechanics to model vol- umetric muscles and deformable bones, for which solutions are found primarily using the Finite Element or Finite Volume method. With this latter approach, representation of muscles and bones are comparatively more accurate and can represent spatially the internal evolution of the components. However, models based on the latter approach usually represent only a limited subset of relevant anatomical structures owing to the difficulty in constructing and simulating such models.
Following the trend initiated by the industry decades ago, computational models are becoming pervasive in regular clinical practice. Such models can be applied throughout the entire clinical pipeline, from diagnosis to rehabilitation through pre-operative plan- ning, intra-operative guidance and outcome evaluation. Yet, a substantial gap remains between the existing computational models and the physiological reality, hindering a suc- cessful clinical translation. Unlike typical purely mechanical systems found in industry, for which prototypes can be built, validating that a biomechanical model can generate similar outputs as the actual human body is a challenging task. There is no perfect correspondence between simulations and experiments. First, the major simplifications required to model such complex system hinders accuracy. Second, it is hardly feasible to gather the tremendous amount of information needed to replicate numerically a specific subject. Finally, there are no conforming test that guarantees the validity of a model in every scenario due to inter-subject variability. Confidence in a model can only be gained statistically through recurring comparisons with available and new data in the literature.
In summary, human movement and its underlying mechanisms are difficult to understand because it is a cerebral action, subject-specific and the actual in-vivo effects are un- measurable without invasive procedures. Musculoskeletal models can overcome these limitations and provide new insights such as via muscle activation, joint reaction forces, range-of-motion etc. However, long-lasting efforts are needed to validate and gain confidence in the predictions generated by these models.
1.1 a nat o m y o f t h e s h o u l d e r 3
Clavicle Scapula
Humerus
Radius Ulna
Scapular Coracoid process Humeral Greater tubercle Humeral head
Humeral Lesser tubercle Humeral Coronoid fossa Humeral Radial fossa Humeral Lateral epicondyle Scapular Acromion
Humeral Capitulum Humeral Medial epicondyle
Humeral Trochlea
LANDMARKS BONES
Figure 1.1:Skeletal anatomy of the shoulder [Betts et al.,2013].
While some joints in the body are extensively covered in the literature, such as the knee or the hip, the shoulder joint is relatively less understood. Among the musculoskeletal subsystems of the human body, the shoulder presents unique challenges owing to its singular kinematics. Indeed, the shoulder is the articulation with the greatest range of motion within the whole body. It is a consequence of minimal bone-to-bone contact and few connective tissues. The shoulder complex is made up of more than twenty muscles, acting in coordination to control the motion of the upper limb and to maintain stability of the articulation. With more than 20 muscles for controlling the position of the elbow, and by extension the hand, the system is highly redundant. As a result, neuromuscular control and muscle recruitment strategy is an important aspect of the dynamic simulation of the upper limb. With the advent of modern medical imaging techniques and numerical modeling approaches, the comprehension of the function of the shoulder joint is increasing. Yet, many aspects are left to explore, in particular, how the active motion is affected by impairments or dysfunctions, and corrective surgeries.
1.1 Anatomy of the Shoulder
The shoulder is made up of 3 bones: the clavicle, the scapula and the humerus. The clavi- cle is connected to the thorax, at the sternum, via the sternoclavicular articulation. The scapula is connected to the clavicle via the acromioclavicular articulation and coracoclav- icular ligaments. Finally, the humerus is connected to the scapula via the glenohumeral articulation, and the capsular and coracohumeral ligaments. 21 anatomical muscles connect the shoulder to the rest of the body. Each muscle is divided into segments, depending on which bone the tendon originates from or inserts onto. Each segment is potentially divided into further functional units, depending on whether nerve endings or the number of tendon branches are considered. However, this last division is the subject of intense research [Smith et al.,2003; Brown et al.,2007; Rispoli et al.,2009; Sakoma et al.,2011; Nasu et al.,2012; Kim et al.,2013; Moccia et al.,2015; Larionov et al.,2018].
4 1 i n t r o d u c t i o n
Figure 1.2:Muscles of the shoulder, adapted from [Betts et al.,2013].
1.2 m o d e l i n g o f t h e s h o u l d e r m u s c l e s 5
1.2 Modeling of the Shoulder Muscles
First known models of the shoulder appeared at the beginning of the 20th century [Mollier, 1899]. These models were mechanical apparatus wherein muscles were represented as strings. Modernized versions of mechanical apparatus are still used nowadays [Favre et al.,2005; Baumgartner et al.,2014], especially to test shoulder prostheses [Gutiérrez et al.,2008; Favre et al.,2010b; Vaupel et al.,2012; Petrillo et al.,2016]. Numerical models of the shoulder emerged in the 90s with modeling choices following those initiated by mechanical apparatus. The mechanical behavior of these unidimensional muscle representations is founded in the long-established Hill’s muscle model [Hill,1938; Zajac, 1989], which captures the force-length and force-velocity relationship of a muscle segment.
Other phenomenological models have also been proposed [Delp et al.,1990; Schutte, 1993; Thelen,2003; Millard et al.,2013]. Another class of mechanical models is inspired by the cross-bridge theory [Eisenberg et al.,1980; Zahalak et al.,1990]. While being derived from the microstructure of muscle, material models based on this approach require many parameters that are difficult to measure. Muscles that are modeled as strings are made to wrap on the bones via intermediate wrapping structures, where the bone surfaces are approximated by analytical geometric primitives such as spheres, cylinders or ellipsoids [Garner et al.,2000; Audenaert et al.,2008; Scholz et al.,2016;
Aurbach et al.,2020]. These wrapping structures are placed and attached to the bones to represent a stable approximate path of the muscle segment during motion. Wrapping on arbitrary triangular surface meshes, which are used to represent bone geometry, has been approached by solving an optimization problem [Marai et al.,2004; Desailly et al., 2010], using contact detection, e.g., using a Finite Element commercial software [Favre et al.,2010a] or the heat method [Zarifi et al.,2017]. A muscle string path is usually further constrained by via-points, which are points fixed in a bone coordinate frame and through which the muscle segment is constrained to pass [Delp et al.,1990; Garner et al.,2001; Carman et al.,2005]. Via-points are needed to prevent a line-segment to snap around the wrapping structure in the wrong direction or to find a numerically feasible but anatomically incorrect path.
Musculoskeletal modeling and simulation have received growing interest in the field of computer graphics, as computer generated images are ubiquitous in movies, video games and other media [Ziva Dynamics; Weta Digital]. Animated characters are an important part of such productions and character’s motions, either fine-scale or large-scale, should be as realistic as possible. Many modeling software come with an implementation of a muscle component that allows to drive an abstract skeleton rig [Lee et al.,2011]. Such simple muscle models allow to visualize physiological limits and more importantly to deform the character representation to mimic visible deformations, e.g., increasing the size of the arm when the biceps is contracted. Creating such models is a time-consuming task and animating those require expertise to yield visually pleasing and plausible results. Therefore, larger scale models with volumetric muscles are of high interest.
Simulation of the muscle contraction is usually performed via on an underlying line- segment representation, which is either one-way or two-way coupled with the volumetric tissue [Teran et al.,2005; Lee et al.,2009; Berranen et al.,2014; Lee et al.,2018b]. As a result, the volumetric representation is a passive blob that reacts based on the external forces computed by the underlying representation. Some aspects of morphing a template
6 1 i n t r o d u c t i o n
Figure 1.3:Various approaches to musculoskeletal modeling. From left to right, the illustrations are from [Chadwick et al.,2014], [Webb et al.,2014], and [Teran et al.,2005].
model into a new one has been investigated, for which medical imaging and motion capture play a major role [Ali-Hamadi et al.,2013; Kadleˇcek et al.,2016]. Such an approach can significantly shorten the development of a new character, even non-humanoids, and offer the possibility of generating a wide range of morphologies. While being visually plausible, the validity of such models based on biomechanical grounds is yet to be proven.
The use of volumetric models in biomechanics is well-established [Zheng et al.,2017].
Unlike the former discussed computer graphics based models, they propose a more targeted anatomical simulation with fewer structures. The usual goal is to acquire understanding of specific phenomena, e.g., tear propagation of a muscle. For the shoulder, the occurrence and evolution of supraspinatus tears is a major topic [Seki et al.,2008;
Inoue et al.,2013; Engelhardt et al.,2016; Quental et al.,2016b]. Muscles included in models of the shoulder joint are restricted to the deltoid and rotator cuff [Astier,2010;
Webb et al.,2014]. The active parts of the muscle material is either embedded within a continuum-based strain energy function [Webb et al.,2014; Röhrle et al.,2017] or as truss connecting the nodes of the volumetric mesh [Hedenstierna et al.,2008; Astier,2010]. Due to the fewer number of structures involved, there are currently no applications focusing on trajectory control of such models.
1.3 Modeling of Shoulder Articulation
In musculoskeletal models, anatomical articulations are usually modeled as kinematic joints. When present, the sternoclavicular and acromioclavicular articulations are modeled as spherical joints in musculoskeletal models [Karlsson et al.,1992; Van der Helm,1994;
Maurel et al.,1996; Garner et al.,1999; Charlton et al.,2006; Blana et al.,2008; Quental et al.,2012; Hölscher et al.,2016; Quental et al.,2016a]. The motion of the scapula can be either set using regression equations [Karlsson et al.,1992; Holzbaur et al.,2005;
Dickerson et al.,2007], or controlled by muscles. Regression equations are convenient
1.4 s h o u l d e r a r t h r o p l a s t y 7
to simulate a scapulothoracic motion when the shoulder girdle structures are absent, although they would substantially affect such motion. When the shoulder girdle muscles are present, the motion of the scapula on the thorax is either constrained (reifying the scapulothoracic joint) orfree-hanging. In many models, two points on the medial border of the scapula are enforced to glide on an ellipsoid which is fitted to the thorax [Van der Helm,1994; Maurel et al.,1996; Garner et al.,1999; Charlton et al.,2006; Blana et al., 2008; Quental et al.,2012; Quental et al.,2016a]. More complex formulations have been recently described: In [Ingram et al.,2016] as two point constraints, where each point is constrained on a different ellipsoid, yielding a 2-3 parallel mechanism; in [Seth et al.,2016]
as a 5-dofs kinematic joint gliding on an ellipsoid fitted to the thorax; in [Niyetkaliyev et al.,2017] as a 6-4 parallel mechanism. There are only a few models with afree-hanging scapula. In [Chadwick et al.,2014], the scapula is maintained around an ellipsoid fitted to the thorax, via a spring such that the spring stiffness is set high when the scapula goes inside the thorax, and null or low when it is outside the thorax. In [Hu et al.,2020], a series of spring-dampers are used to maintain the scapula on an ellipsoid fitted to the thorax.
The glenohumeral joint, or more commonly named the shoulder joint, is the principal articulation of interest in the shoulder. It is commonly modeled as a ball-socket kinematic joint in large-scale musculoskeletal models [Karlsson et al.,1992; Van der Helm,1994;
Maurel et al.,1996; Garner et al.,1999; Holzbaur et al.,2005; Charlton et al.,2006;
Dickerson et al.,2007; Blana et al.,2008; Quental et al.,2012]. While conceptually correct, this design choice neglects the translation of the humeral head within the glenoid. Indeed, the glenoid is a narrow cup, close to a plane, and the radii of both the glenoid and the humeral head do not normally match, nor the curvature remains constant across their surfaces. Translation of the humeral head within the glenoid is a major variable to analyze the stability of the joint. As a result, recent studies have been developing newer formulations of the glenohumeral joint to include the extra translational degrees of freedom above. In [Terrier et al.,2007; Terrier et al.,2008], the non-penetration between the humerus and the scapula is enforced via contacts mechanics, while in [Favre et al., 2012], the humerus is initially constrained and progressively relaxed as long as the muscle load sharing problem can find a solution. However, these two models in the literature are limited to the glenohumeral joint, with only the rotator cuff and deltoid muscles present.
In comprehensive musculoskeletal models, a relaxed glenohumeral joint was recently modeled as a compliant constraint (i.e. a spring) in [Charbonnier et al.,2014], or as a sphere-sphere joint in [El Habachi et al.,2015; Quental et al.,2016a].
1.4 Shoulder Arthroplasty
Shoulder arthroplasty is a standard clinical procedure for the treatment of various pathologies such as arthropathy, osteoarthritis, or rotator cuff tears [Drake et al.,2010;
Ricchetti et al.,2011]. It consists of replacing the glenoid and the head of the humerus by a prosthesis. Surgeons have the possibility to implant either so-called anatomical or reverse implants, which are illustrated inFigure 1.5. The former is usually indicated for patients with a functioning rotator cuff, whereas the latter was specifically intended for patients with major rotator cuff tears (MRCT) [Flatow et al.,2011; Mattei et al.,2015]. Unlike the anatomical version, a reverse shoulder arthroplasty (RSA) is easier to insert and remain
8 1 i n t r o d u c t i o n
Figure 1.4: Total Shoulder Arthroplasty (left) and Reverse Total Shoulder Arthroplasty (right).
Used with permission of Mayo Foundation for Medical Education and Research, all rights reserved.
tight over the long term [Casagrande et al.,2016; Gregory et al.,2017]. In addition, RSA is the ultimate salvage procedure after any complications or for a revision surgery. In particular, an anatomical implant is usually converted into a reverse one in response to general deterioration of the rotator cuff muscles, which is a common complication of the initial procedure [Walker et al.,2012; Abdel et al.,2013; Alentorn-Geli et al.,2015;
Hernandez et al.,2017]. Since the conversion from an anatomical implant to a reverse one is a complex procedure with non-negligible rate of failure, RSA is becoming the go-to surgical procedure even when the rotator cuff muscles are in good condition [Palsis et al., 2018].
1.5 Muscle Transfer
Rotator cuff tears are one the main conditions affecting the shoulder and requiring surgical intervention. These are typically tendon snapping off near their attachment site onto the bones. Normally the solution considered first is to attempt re-attaching the torn muscles’ tendons on its original attachment location. Unfortunately this is not always
1.6 t h e s i s g oa l s 9
possible, due such as to extensive damage or the muscles shortening and stiffening until the time to surgery. Absence of the ruptured muscles may reduce the mobility of the shoulder, resulting in restricted range-of-motion, pain, difficulties in performing common daily life activities and thus reduced quality of life [Gumina et al.,2017; Minagawa et al., 2013]. Arthroplasty is a potential option, but it is a destructive and extreme solution, especially for younger patients. Muscle transfer presents itself as an alternative solution for preserving the articulation. It involves transferring the attachment of a fully capable muscle onto the insertion area of the torn muscle’s tendon, aiming to restore the function of the ruptured muscle. [Elhassan et al.,2010; Omid et al.,2013; Shin et al.,2016; Axe,2016;
Clark et al.,2018]. Choice of muscle to transfer depends on the type of tears impairing the rotator cuff. Tears of the rotator cuff can be divided into two groups, on one hand ruptures affecting the posterior part of the humerus head, i.e. touching the infraspinatus, potentially along with the teres minor and/or the supraspinatus, and on the other hand conditions affecting the anterior part of the humerus head, i.e. touching the subscapularis, often along with the supraspinatus. For ruptures on the posterior side of the humerus head, the main muscle of choice for the transfer is the latissimus dorsi [Gerber,1992; Aoki et al.,1996; Miniaci et al.,1999; Schoierer et al.,2001; Warner et al.,2001; Gerber et al., 2006; Iannotti et al.,2006; Nové-Josserand et al.,2009; Weening et al.,2010; Gerber et al., 2013], while attempts have been made with the teres major [Celli et al.,1998; Schoierer et al.,2001; Henseler et al.,2013; Kolk et al.,2018]. A recent technique involving the lower trapezius has shown great promises for infraspinatus deficiency [Gracitelli et al., 2014; Elhassan,2014a; Elhassan et al.,2016; Stoll et al.,2019]. For ruptures on the anterior side, principally involving the subscapularis, the muscle of choice is the pectoralis major, either in full or some segments [Resch et al.,2000; Jost et al.,2003; Jennings et al.,2007;
Gavriilidis et al.,2010; Nelson et al.,2014; Valenti et al.,2015; Gavriilidis et al.,2010; Shin et al.,2016; Ernstbrunner et al.,2019]. Some anecdotal attempts have been performed with the pectoralis minor in clinical studies [Wirth et al.,1997; Paladini et al.,2013], and with the combined latissimus dorsi and teres major in an anatomical feasibility study [Elhassan et al.,2014b]. The main corpus of studies concerns the choice of segments within the pectoralis major, e.g. clavicular or sternal, and the technique, e.g. sub-coracoid or sup- coracoid. Biomechanical studies on the topic are relatively rare with respect to clinical studies. Most of them focus on muscle transfer for posterosuperior tears [Magermans et al.,2004b; Magermans et al.,2004a; Favre et al.,2008; Ling et al.,2009; Oh et al.,2013;
Omid et al.,2015] comparatively than for anterior tears [Konrad et al.,2007; Jastifer et al., 2012]. Consequently, the muscle transfer procedure follows a trial-and-error approach rarely substantiated by prior biomechanical studies. The outcome of the procedure is ranging from mixed to positive depending on the considered scenario [Omid et al.,2013;
Axe,2016; Clark et al.,2018].
1.6 Thesis Goals
Given the above background on previous musculoskeletal modeling of the shoulder and motivated by the needs in healthcare to better understand the in-vivo generation of human movement, intact or otherwise, computational models are an essential tool to comprehend the interplay of complex internal interactions during complex movements, with potential clinical translation in many application settings. The goal of this thesis is to develop modern modeling techniques for the musculoskeletal simulation of the
10 1 i n t r o d u c t i o n
Figure 1.5:Intact anatomy (left), pectoralis major transfer of the clavicular part (middle) and sternal part (right). Illustrations of the transfer from [Talbot et al.,2013].
shoulder, attempting to reach a middle-ground between fast multi-body dynamics models and complex Finite Element models, and to validate and apply these to enhance the biomechanical knowledge underlying human movement, in either intact, pathological, or post-surgical conditions. To that end we use shoulder arthroplasty and muscle transfer as two demonstrative surgical scenarios.
1.7 t h e s i s o u t l i n e a n d c o n t r i b u t i o n s 11
1.7 Thesis Outline and Contributions
The contributions and the structure of the thesis are outlined below:
Instead of the conventional line-segment approach to model muscles, a comprehensive volumetric Finite Element musculoskeletal model of the shoulder is presented inChapter 2.
Time is an important factor for optimization based workflow, as well as in clinical settings. Taking the time constraint into consideration, muscle geometries are modeled as simplified hexahedral slabs, allowing a globally low number of elements in order to reduce simulation time from hours to minutes. Moment arms of the rotator cuff and deltoid muscles generated by the model are validated against the biomechanical literature, which includes a detailed Finite Element model [Webb et al.,2014] and a mechanical apparatus [Ackland et al.,2008], for abduction and internal rotation. Unlike most models, the dynamics of the scapula in the model is not enforced by regression equations nor by a kinematic joint formulation, but enabled through the shoulder girdle muscles and contact with the thorax. Given this feature, scapular winging, a pathology affecting scapula kinematics, is simulated and compared to clinical knowledge. This work has been published in a peer-reviewed journal as [Péan et al.,2019].
Owing to the observation that many muscles of the shoulder are relatively flat, a novel approach to model muscles based on thin-shell representation is described inChapter 3.
Furthermore, a method to generate muscle segment models based on curves where the tendons attach to the bones is proposed, which overcomes the difficulty of generating 3D models experienced first-hand inChapter 2. The surface-based modeling choice is compared qualitatively and quantitatively to alternative line-segment and volumetric muscle representations. The robustness of the model is tested on complex trajectories obtained from motion capture, which reproduces several activities of daily living. Paraly- sis of the infraspinatus muscle is simulated and compared to the intact model based on clinical knowledge. This work has been published in a peer-reviewed journal as [Péan et al.,2020c].
InChapters 4and5, the model developed in the previous chapter is used as the funda- mental block for answering biomechanical questions relevant to current clinical practices.
InChapter 4, Reverse Shoulder Arthroplasty is studied in simulations for various degrees of rotator cuff tears. The main goal is to assess the forces going through the joint with (near)-intact rotator cuff muscles with respect to more severe tears. Anterior flexion of the arm is simulated, which eases the validation with respect to prior knowledge and in-vivo data. The model compares favorably with the observations reported the literature, noticeably: joint reaction forces increasing at higher angle of anterior flexion similarly in-vivo measurements, and scapular compensation with massive rotator cuff tears. With a reverse arthroplasty, joint compressive forces with an intact or near-intact rotator cuff are shown to be up to more than two times the force than with a massive rotator cuff tears.
Consequently, it may lead to an increased wear of the prosthesis. As reverse arthroplasty becomes more commonly the first choice of surgery regardless of the integrity of the rotator cuff, such wear might impact the rate of revision surgery, which is particularly relevant when performing a reverse arthroplasty on a younger population. This work is currently under review as [Péan et al.,2020b].
12 1 i n t r o d u c t i o n
InChapter 5, analysis of the pectoralis major transfer in response to isolated tear of the subscapularis is studied in simulations. The pectoralis major transfer considered herein involves attaching either the clavicular, sternal, or both parts, onto the former attachment site of the subscapularis. The muscles activation generated by the inverse controller are compared to recorded EMG measurements. Differentiating itself from previous works, our study presents a comprehensive analysis of glenohumeral joint forces as well as muscles activation, during complex motion of activities of daily living. It is shown that while the subscapularis and pectoralis major, particularly the calvicular segment, are considered synergistic, they have asynchronous activation patterns prior transfer. After the transfer, activation patterns of the pectoralis major change significantly. Such results may provide new information on why some patients have difficulty adapting to the post-surgical muscular configuration, and might make clinicians to include biofeedback exercises more commonly in rehabilitation programs. Moreover, the transfer leads to a noticeable change of patterns in surrounding muscles, where the intact remaining rotator cuff muscles are partially restored to pre-tear condition, while greatly increasing the effort required from the anterior part of the deltoid. Such increase is aligned with clinical recommendations whereby a capable anterior deltoid is a strong prerequisite for the success of the surgery. In a broader scope, this study demonstrates the application of computational models to study intrinsic phenomena such as muscle activations, on daily life scenarios, beyond in-plane anatomical motion – which is hardly feasible with in-vivo and ex-vivo models. This work is currently under review as [Péan et al.,2020a].
This thesis is concluded inChapter 6with brief summary of the proposed methods and contributions, and a discussion on potential future research directions.
2
A Comprehensive and Volumetric Musculoskeletal Model for the
Dynamic Simulation of the Shoulder Function
We present a volumetric and extensive finite element model of the shoulder usable in the context of inverse control, in which the scapula is left unconstrained on the ribcage.
Such a model allows for exploring various shoulder movements, which are essential for making patient-specific decisions. The proposed model consists of 23 volumetric muscles parts modeled using the finite element method. The glenohumeral, acromioclavicular and sternoclavicular joints are modeled with soft ball-socket constraints. The musculoskeletal model can be controlled by a tracking-based algorithm, finding the excitations values in the muscles needed to follow some target points. The moment arms obtained during abduction and rotation are compared with the literature, which includes results from cadaveric data and a fine FE model of the rotator cuff and the deltoid. We simulated the paralysis of serratus anterior, a main reason of scapular winging, and compared it with its physiological counterpart. A deficiency in the range of motion as well as a reduction in upward rotation were observed, which both corroborate clinical observations. This is one of the most comprehensive models of the shoulder, which can be used to study complex pathologies of the shoulder and their impact on functional outcome such as range-of-motion.
2.1 Introduction
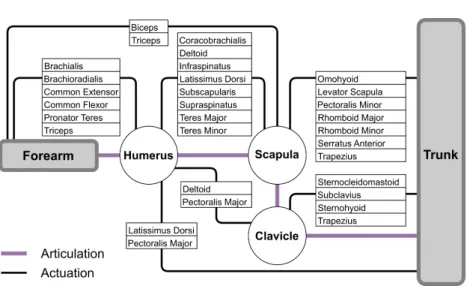
Many musculoskeletal models of the shoulder have been proposed in the literature over the past decades. These models typically represent muscles by a set of lines [Chadwick et al.,2014; Van der Helm,1994; Dickerson et al.,2007; Charlton et al.,2006], having the primary advantage of being simple to simulate and computationally efficient. However,
This chapter has been published as the following journal article: Fabien Péan, Christine Tanner, Christian Gerber, Philipp Fürnstahl, Orcun Goksel, "A Comprehensive and Volumetric Musculoskeletal Model for the Dynamic Simulation of the Shoulder Function", Computer Methods in Biomechanics and Biomedical Engineering 22(7), pp. 740–751, 2019.
https://doi.org/10.1080/10255842.2019.1588963.
13
14 2 a c o m p r e h e n s i v e a n d v o l u m e t r i c m u s c u l o s k e l e ta l m o d e l. . .
Figure 2.1:Connectivity diagram of the musculoskeletal system of the shoulder.
limitations of line models have been repeatedly reported due to their inability to correctly capture moment arms, fiber arrangements of muscles, and volumetric interactions of muscles with bones and other muscles.
The shoulder is a complex assembly of muscles and bones known to provide the greatest range-of-motion in the human body. It is made up of three bones: humerus, clavicle, and scapula. The last one is unique in that it is loosely connected to the trunk, while sliding freely on the back. The scapula is actuated by many muscles that ensure the force transfer from the arm to the trunk.Figure 2.1describes interdependencies in this musculoskeletal system by listing muscles that have a direct influence on the bones of the shoulder. In previous line-segment models, the scapula is usually constrained on the trunk through heuristic means, such as using numerical constraints [Seth et al.,2016; Blana et al.,2008], or a regression equation [Holzbaur et al.,2005; Xu et al.,2015]. This may artificially limit the range-of-motion, and prevents the study of pathologies related to the scapula.
Considering the bones as rigid bodies is a standard hypothesis in movement science, since during daily activities the bones do not deform sufficiently to influence the motion. On the other hand, muscles require detailed modeling and numerical treatment to simulate their deformations, actions, and incorporate their properties, such as shear force transfer and muscles fiber directions. The standard approach using line-segment models requires numerous fibers to simulate broad-attachment muscles. This can model neither any shear force transfer between the fascicles, nor any volumetric deformation.
The Finite Element (FE) method is a robust and well-known approach used for simulation of deformable objects. Muscles can be represented as volumes in the model, allowing to overcome the aforementioned limitations posed by line-segment models. FE models
2.1 i n t r o d u c t i o n 15
have been used thoroughly to study the shoulder [Zheng et al.,2017; Webb et al.,2014].
However, most works in the literature consider only a limited number of muscles, which cannot be representative of the complex shoulder and faithfully model its motion, and without any active control through inverse-dynamics. The modeling of active contraction of fibers in a FE model can be incorporated by choosing an appropriate constitutive model of the muscle material integrating the active contraction part in the 3D continuum level [Blemker et al.,2005; Weickenmeier et al.,2014]. The fibers directions are then discretized at each integration point of the element during the assembly procedure. The alternative approach consists in using separate discretizations for the fibers and its embedding tissue matrix [Berranen et al.,2014; Hedenstierna et al.,2008]. In this approach, fibers are usually defined individually as 1D wire-segments using the three-element Hill muscle model, surrounded by a volumetric FE mesh. Many fiber directions are integrated per element. When no DTI image is available to obtain specific fiber directions, solving the heat equation on the underlying FE mesh between muscle origin and insertion can offer an approximation of fiber directions throughout muscles [Choi et al.,2013]. Several muscle models above, such as springs, embedded springs or continuum based materials, are implemented in the powerful and flexible multi-body biomechanical simulation framework,Artisynth[Lloyd et al.,2012], which we utilize in this work. Artisynth allows for coupling rigid (bone) models with deformable (muscle) models, which can also be activated and kinematics-controlled.
Main technical limitations for the extension and generalization of musculoskeletal FE models are (i) the difficulty of creating models of larger systems, (ii) the computation time of the simulations when using detailed finite element models, and (iii) the complexity of collision handling, especially, with increasing number of musculoskeletal bodies. Such limitations have prevented the analysis of any complex movement, e.g., involving the scapula, limiting the utility of such models. The limitations above are also among the reasons for the absence of such models in a clinical pipeline for surgical planning, which would require fast computational times to allow for trial-and-error or optimization approaches, while maintaining high-fidelity.
The forward simulation of the upper limb based on continuum mechanics has been approached by [Röhrle et al.,2017]. The study focused on a two-muscles system with antagonistic actions. However, when considering the shoulder mechanism as a whole, many muscles are needed to perform a single motion due to the limited mechanical constraints in between the bones that gives to the shoulder the greatest range-of-motion in the body.
Our study aims to (i) present a sophisticated FE model of the shoulder mechanism used in an inverse control context for the study of motion and (ii) to demonstrate the utility of our model in a clinical pipeline by simulating a pathological condition of the scapula.
16 2 a c o m p r e h e n s i v e a n d v o l u m e t r i c m u s c u l o s k e l e ta l m o d e l. . .
2.2 Methods
2.2.1 Geometric model
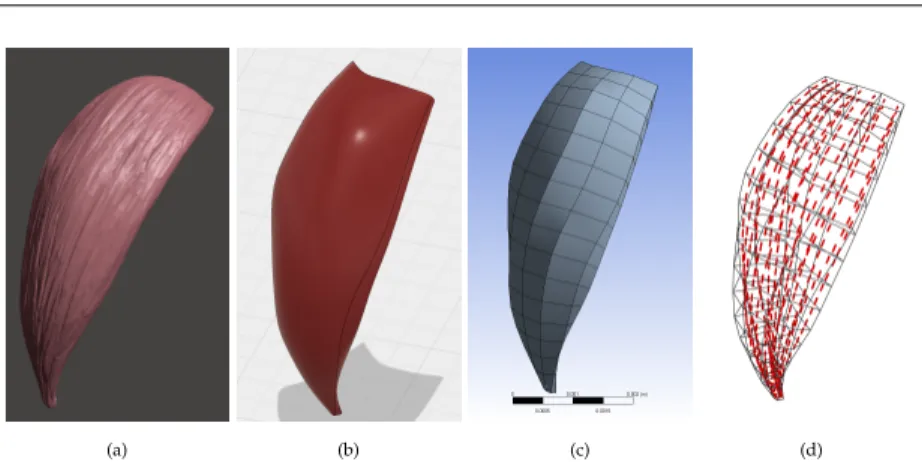
To construct our shoulder model, we used the dataset BodyParts3D©(The Database Center for Life Science, Japan). This dataset consists of a collection of surface meshes representing muscles, bones, and other structures, seeFigure 2.2a. We constructed the 3D volumetric mesh models for the FE simulations from these surface meshes. For reasonable simulation time in an inverse context, the number of elements and hence the mesh resolution is crucial. The meshes of the muscles were crafted carefully in the CAD software named Autodesk® Fusion 360™ using smooth geometric approximations, by discarding any negligible minute details in the input meshes, seeFigure 2.2b. These surface models were then meshed with trilinear hexahedral elements in ANSYS®Workbench r17.0, see Figure 2.2c. A list of the muscles included in our model is given inTable 2.1.
The muscle fiber directions were computed following the approach detailed in [Choi et al.,2013]. The problem is stated as solving the heat equation as follows:
∆ϕ=0 ∀ϕ∈Ω ϕ=−1 ∀ϕ∈∂Ωorigin ϕ= +1 ∀ϕ∈∂Ωinsertion
(2.1)
over the muscle domainΩfor a dummy variableϕwith boundary conditions imposed on the nodes located at the junction between the muscle and the bones, i.e. -1 at the origin and +1 at the insertion. The fiber direction at any point of the FE domain can be obtained from the gradient of the scalar field∇ϕ(x),∀x∈Ω, seeFigure 2.2d.
Bones were modeled as rigid bodies with triangular surface meshes, based directly on the BodyParts3D dataset. This neglects any deformation within the bones, which is sufficient approximation in comparison to the large musculoskeletal motions considered herein.
Figure 2.3shows our complete model from front, side, and back views.
Wrapping is a technique used in line-segment models to enforce the path of a string (e.g. ligaments). It commonly works only for interactions with simple geometrical objects (e.g. spheres and cylinders) acting as a surrogate to the intended anatomical structure (e.g. cylinder for the shaft of the humerus). Wrapping of actual anatomical structures, having more complex geometries, therefore requires carefully aligning one or more of these simple geometric shapes, which is a tedious modeling and inaccurate simulation process. In our model, the bone surfaces themselves are used to test for collisions and accordingly muscle wrapping. The constraints resulting from confirmed intersections between triangular surface mesh pairs are resolved through the use of Lagrange multipliers. No wrapping object needs to be adjusted to the model, which is advantageous in clinical settings to reduce patient-specific model setup, and also more accurate as no errors are introduced by uncertainty on the position or shape of the wrapping object. Collisions tests are computationally burdensome. Thus to minimize unnecessary tests in our simulations, we individually enabled or disabled
![Figure 1.3: Various approaches to musculoskeletal modeling. From left to right, the illustrations are from [Chadwick et al., 2014], [Webb et al., 2014], and [Teran et al., 2005].](https://thumb-eu.123doks.com/thumbv2/1library_info/5289416.1676802/24.629.86.544.119.340/figure-various-approaches-musculoskeletal-modeling-illustrations-chadwick-teran.webp)

![Figure 2.4: (a) Dynamic Arm Simulator of [Chadwick et al., 2014] showing that an ellipsoid may not be sufficient to geometrically approximate the ribcage](https://thumb-eu.123doks.com/thumbv2/1library_info/5289416.1676802/37.629.93.551.114.275/figure-dynamic-simulator-chadwick-ellipsoid-sufficient-geometrically-approximate.webp)
![Figure 2.5: Moment arms (cm) from our simulation of shoulder abduction in frontal plane (bold lines) overlaid on [Webb et al., 2014] for a thoracohumeral angle ranging from 0 to 90°](https://thumb-eu.123doks.com/thumbv2/1library_info/5289416.1676802/41.629.100.541.114.384/figure-moment-simulation-shoulder-abduction-frontal-overlaid-thoracohumeral.webp)
![Figure 2.6: Moment arms (cm) from our simulation of shoulder axial rotation (bold lines) overlaid on [Webb et al., 2014]](https://thumb-eu.123doks.com/thumbv2/1library_info/5289416.1676802/42.629.87.530.101.384/figure-moment-simulation-shoulder-axial-rotation-lines-overlaid.webp)
![Figure 2.7: Moment arms (cm) of shoulder abduction in scapular plane. Comparison from our simulation (solid lines) with ex-vivo data from [Ackland et al., 2008] (dotted lines)](https://thumb-eu.123doks.com/thumbv2/1library_info/5289416.1676802/43.629.105.529.115.353/figure-moment-shoulder-abduction-scapular-comparison-simulation-ackland.webp)

