Z. Kristallogr. NCS 219 (2004) 59-60
© by Oldenbourg Wissenschaftsverlag, München
59
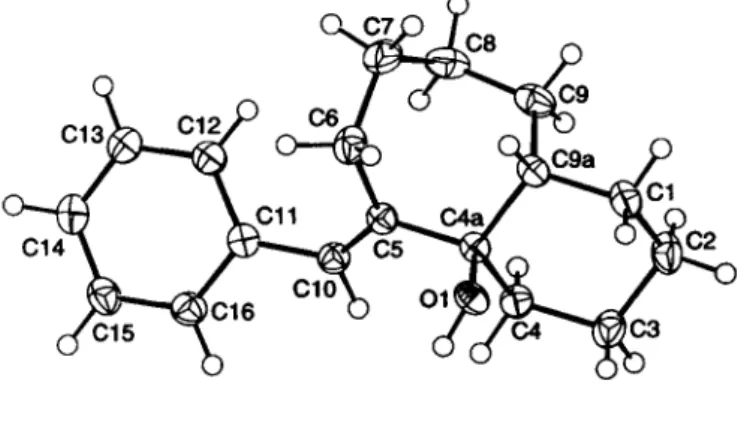
Crystal structure of c«-(5E)-phenylmethylidenedecahydro-4a//-benzo[a]- cyc!ohepten-4a-ol, C18H24O
A. Hölemann
1,1. Brüdgam", H. Hartl*
nand H.-U. Reißig
1' Freie Universität Berlin, Institut für Chemie - Organische Chemie, Takustr. 3, D-14195 Berlin, Gennany
" Freie Universität Berlin, Institut fiir Chemie - Anorganische Chemie, Fabeckstr. 34-36, D-14195 Berlin, Gennany Received January 21, 2004, accepted and available on-line February 9, 2004; CCDC no. 1267/1212
Table 1. Data collection and handling.
Abstract
C18H24O, tetragonal, PAiln (no. 86), a = 13.885(2) A, c = 14.833(3) A , V = 2859.7 A
3, Z = 8,
R
gt(F) = 0.044, wR^F
2) = 0.129, T= 173 K.
Source of material
The title compound was obtained by samarium diiodide-induced cyclization of 2-(6-phenylhex-5-ynyl)cyclohexanone according to the procedure described in [1,2]. The crude mixture was puri- fied by chromatography on silica gel followed by HPLC separa- tion of the two isomers; m.p. 337-338 K.
Discussion
The constitution and configuration of the tide compound were un- ambiguously determined by the crystal structure. Whereas the 7- membered ring adopts a boat conformation, the 6-membered ring exists as expected in a chair conformation. The hydroxy group is placed in an axial and the bridgehead hydrogen atom in an equato- rial position proving the cis-fusion of the two rings. Bonding dis- tances and bond angles show normal values.
Crystal: colorless block, size 0.1 x 0.4 x 0.5 mm Wavelength: Mo Ka radiation (0.71073 A)
/*• 0.71 cm"1
Diffractometer, scan mode: Broker SMART CCD, <fi/(o with tsto = 0.3°
7ßnux" 61.14°
N(hkl)measaad, N(hkl)uniquc- 34781,4384
C r i t e r i o n f o r /obs, N(hkl)gl: l<*R>lo(IdKh 3238
N(param)rcfmed-. 268
Programs: SHELXS-97 [3], SHELXL-97 [4], PLATON [5]
Table 2. Atomic coordinates and displacement parameters (in Ä2).
Atom Site X y z (/ho
H(l) 8« 0.325(1) 0.338(1) 0.243(1) 0.052(5) H(ll) 8« 0.220(1) 0.547(1) 0.297(1) 0.036(4) H(11A) 8« 0.209(1) 0.646(1) 0.243(1) 0.037(4) H(21) 8g 0.340(1) 0.653(1) 0.349(1) 0.049(4) H(22) 8g 0.377(1) 0.672(1) 0.248(1) 0.038(4) H(31) 8« 0.392(1) 0.491(1) 0.342(1) 0.041(4) H(32) 8g 0.481(1) 0.558(1) 0.317(1) 0.046(4) H(41) 8g 0.470(1) 0.428(1) 0.213(1) 0.038(4) H(42) 8g 0.457(1) 0.527(1) 0.160(1) 0.036(4) H(61) 8 g 0.191(1) 0.378(1) 0.070(1) 0.035(4) H(62) 8g 0.244(1) 0.336(1) -0.017(1) 0.035(4) H(71) 8g 0.154<1) 0.509(1) -0.012(1) 0.046(4) H(72) 8s 0.223(1) 0.469(1) -0.094(1) 0.050(5) H(81) 8g 0.359(1) 0.540(1) -0.022(1) 0.041(4) H(82) 8g 0.279(1) 0.620(1) -0.054(1) 0.048(4) H(91) 8 g 0.347(1) 0.643(1) 0.098(1) 0.047(4) H(92) 8« 0.233(1) 0.658(1) 0.084(1) 0.044(4) H(9A) 8« 0.195(1) 0.510(1) 0.1478(9) 0.028(3) H(10) ig 0.475(1) 0.361(1) 0.090(1) 0.036(4) H(12) 8g 0.357(1) 0.381(1) -0.122(1) 0.047(4) H(13) 8g 0.390(1) 0.287(1) -0.253(1) 0.042(4) H(14) 8 g 0.490(1) 0.150(1) -0.239(1) 0.042(4) H(15) 8« 0.559(1) 0.109(1) -0.099(1) 0.043(4) H(16) 8g 0.526(1) 0.206(1) 0.029(1) 0.036(4)
* Correspondence author (e-mail: haitl@chemie.fii-berlin.de)
60
C i8H24 0Table 3. Atomic coordinates and displacement parameters (in Ä2).
Atom Site X y z i / n 1/22 1/33 i/|2 t/13 Un
O(l) 8 g 0.28510(6) 0.38684(6) 0.23183(5) 0.0248(4) 0.0215(4) 0.0324(4) 0.0007(3) 0.0055(3) 0.0041(3) C(l) 8« 0.25183(8) 0.59061(9) 0.25121(8) 0.0262(5) 0.0254(5) 0.0398(6) 0.0019(4) -0.0002(4) -0.0071(5) C(2) 8g 0.34899(9) 0.62302(9) 0.2879(1) 0.0316(6) 0.0306(6) 0.0457(7) -0.0005(5) -0.0044(5) -0.0149(5) C(3) 8g 0.41735(9) 0.5376(1) 0.29464(9) 0.0277(6) 0.0378(6) 0.0362(6) 0.0015(5) -0.0071(5) -0.0112(5) C(4) 8g 0.42711(8) 0.48465(8) 0.20474(8) 0.0195(5) 0.0282(5) 0.0311(5) -0.0005(4) -0.0026(4) -0.0042(4) C(4a) 8« 0.32965(7) 0.45160(7) 0.16734(7) 0.0185(4) 0.0195(4) 0.0252(4) -0.0022(3) 0.0007(3) 0.0005(4) C(5) 8g 0.33590(7) 0.39775(7) 0.07733(7) 0.0213(5) 0.0203(5) 0.0266(5) -0.0029(3) 0.0001(4) 0.0000(4) C(6) 8g 0.24257(8) 0.39180(9) 0.02549(8) 0.0231(5) 0.0363(6) 0.0355(6) -0.0016(4) -0.0042(4) -0.0096(5) C(7) Sg 0.2205(1) 0.4854(1) -0.02862(9) 0.0354(7) 0.0498(8) 0.0365(7) 0.0129(6) -0.0126(5) -0.0067(6) C(8) 8g 0.2916(1) 0.5667(1) -0.01066(9) 0.0406(7) 0.0397(7) 0.0367(6) 0.0080(6) -0.0015(5) 0.0140(5) C(9) 8g 0.28430(9) 0.60889(9) 0.08446(9) 0.0306(6) 0.0247(5) 0.0436(6) -0.0013(4) -0.0037(5) 0.0079(5) C(9a) 8g 0.26004(7) 0.53788(7) 0.16055(7) 0.0187(4) 0.0202(5) 0.0331(5) -0.0010(4) -0.0014(4) -0.0002(4) C(10) 8« 0.41828(8) 0.35712(8) 0.04950(7) 0.0240(5) 0.0256(5) 0.0247(5) 0.0001(4) -0.0002(4) 0.0000(4) C ( l l ) 8« 0.43594(7) 0.30052(8) -0.03312(7) 0.0219(5) 0.0248(5) 0.0271(5) -0.0022(4) 0.0025(4) -0.0006(4) C(12) 8g 0.39693(9) 0.32354<9) -0.11720(8) 0.0354(6) 0.0281(5) 0.0287(5) 0.0038(5) 0.0008(4) 0.0008(4) C(13) 8g 0.4169(1) 0.26844(9) -0.19287(8) 0.0410(7) 0.0356(6) 0.0265(5) 0.0028(5) -0.0002(5) -0.0013(5) C(14) 8« 0.47641(9) 0.18877(9) -0.18613(8) 0.0352(6) 0.0324(6) 0.0327(6) -0.0004(5) 0.0030(5) -0.0071(5) C(15) 8g 0.51623(9) 0.16508(9) -0.10391(9) 0.0294(6) 0.0305(6) 0.0395(6) 0.0045(5) 0.0017(5) -0.0043(5) C(16) 8g 0.49724(8) 0.22079(9) -0.02847(8) 0.0248(5) 0.0326(6) 0.0304(5) 0.0031(4) -0.0004(4) 0.0000(4)
Acknowledgments. Support of this work by the Volkswagen-Stiftung, the Schering AG and the Fonds der Chemischen Industrie is gratefully acknowledged.
References
1. Nandanan, E.; Dinesh, C. U.; Reissig, H.-U.: Competition between Novel 8-endo-dig and 6-trig Cyclizations of Samarium Ketyls Leading either to Benzannulated Cyclooctene or to Hexahydronaphthalene Derivatives.
Tetrahedron 56 (2000) 4267-4277.
2. Hölemann, A.: Intra- und Intermolekulare Samariumketyl-Alkin-und Alien-Kupplungen - Erste Anwendungen in der Synthese mittelgroßer Ringsysteme. Dissertation, Freie Universität Berlin (2004).
3. Sheldrick, G. M.: Phase Annealing in SHELX-90: Direct Methods for Larger Structures. Acta Crystallogr. A46 (1990) 467-473.
4. Sheldrick, G. M.: SHELXL-97. Program for the Refinement of Crystal Structures. University of Gottingen, Germany 1997.
5. Spek, A. L.: PLATON - A Multipurpose Crystallographic Tool. Utrecht University, The Netherlands 1998.