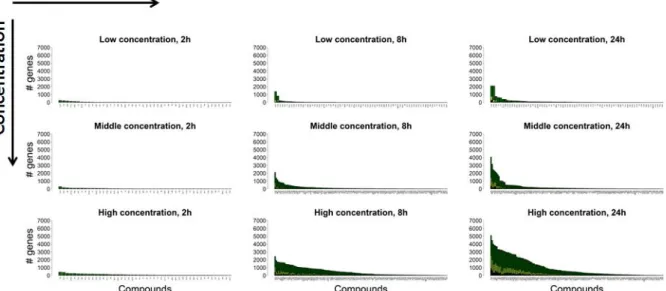
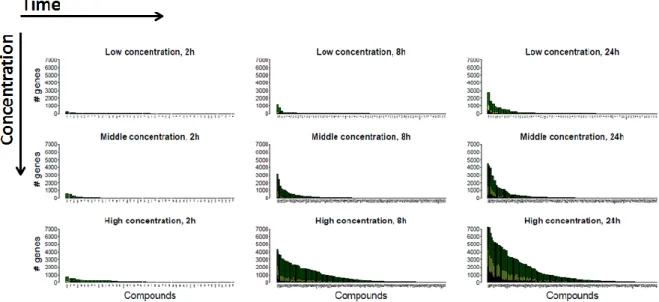
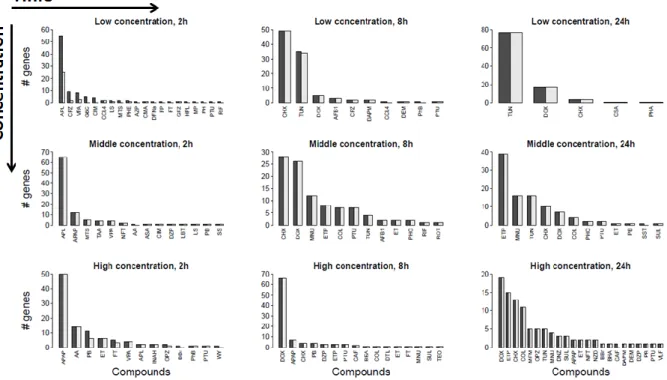
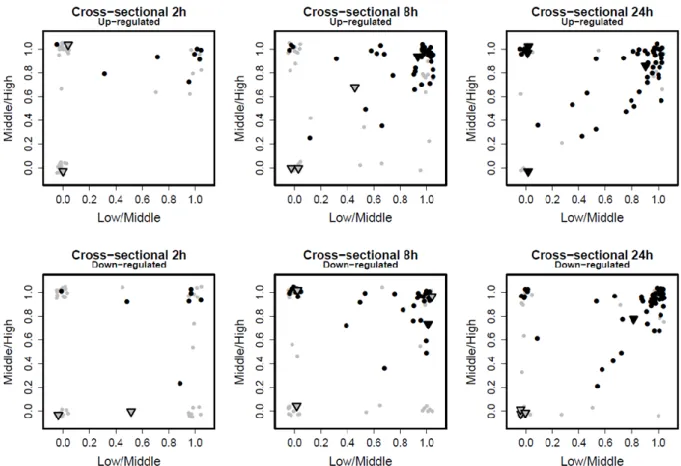
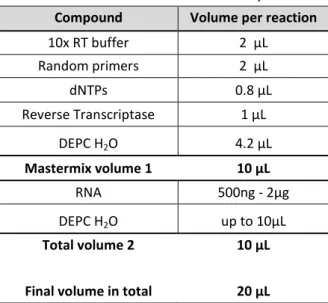
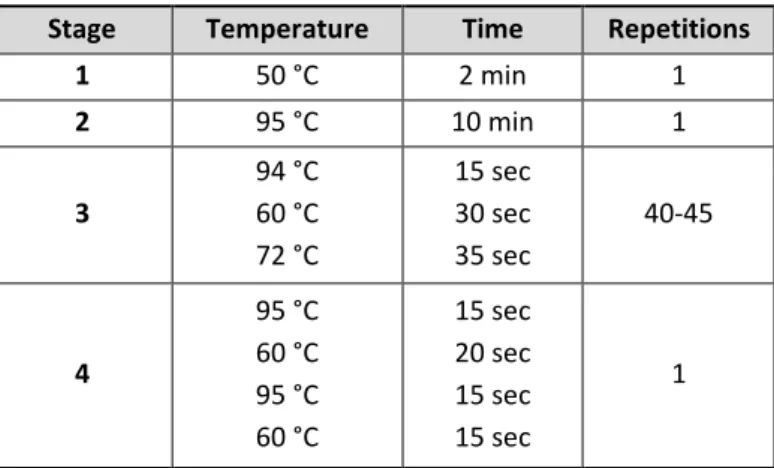
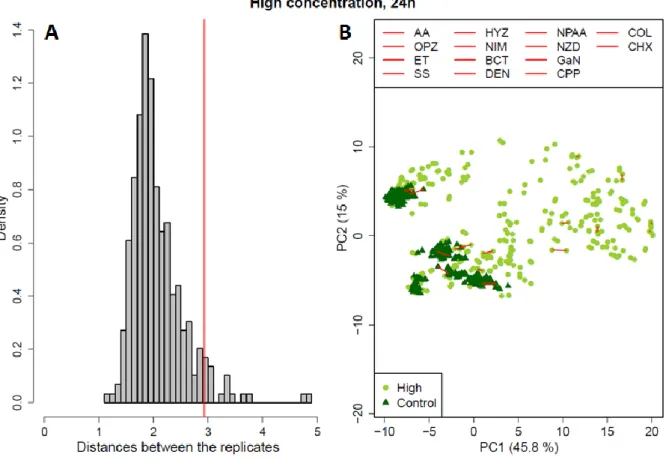
Transcriptomics-based prediction of human hepatotoxic blood concentrations of chemicals.
Volltext
Abbildung

ÄHNLICHE DOKUMENTE
Several studies already proved that ESCs are suitable for the in vitro derivation of male gametes using different strategies. Since it was shown that mouse SSCs
way are the steroid 5α‐reductase type 1 (SRD5A1) and the aldo‐keto reductase 1C2 (AKR1C2). A
This PhD thesis was undertaken to investigate functional TRPM8 and TRPV1 expression in human conjunctival epithelial cells (HCjEC) and ocular tumor cells such as uveal
1) To establish robust methods for isolation of human meniscus cells from native meniscus tissue and for in vitro cell expansion. To investigate their cell morphology
The review with the title “Application of New Cellular and Microphysiological Systems to Drug Metabolism Optimization and Their Positioning Respective to In Silico Tools” (195)
Sorensen (2009) Differences in gene expression of granulosa cells from women undergoing controlled ovarian hyperstimulation with either recombinant follicle-stimulating hormone
The amino acid at position 226, according to H3 numbering, has changed from glutamine (Q) to lysine (L), which is known to be important for the binding preference of human
the principle finding of the cPPIAs strongly supports the finding that MC congeners have comparable PP inhibiting capacities in human cell lines (HEI<293) as well as