ζ . Kristallogr. NCS 213 (1998) 4 6 5 - 4 6 6 465
© by R. Oldenbourg Verlag, München
Crystal structure of α-ytterbium pentaphosphide, a-YbPs and of ß-ytterbium pentaphosphide, ß-YbPs
H. G. von Schnering, M. Wittmann and K. Peters
Max-Planck-Institut für Festkörperforschung, Heisenbergstraße I. D-70569 Stuttgart, Germany
Received December 17, 1997, transferred to 2nd update of database ICSD in 1998, CSD-No. 409179 and CSD-No. 409180
a-YbP,
1. a-Ytterbium pentaphosphide, a-YbPsSource of material: a-YbPs was formed as a majority product (see ß-YbPs) by reaction of the elements in a sealed glass ampoule at 793 К (2 weeks) in the presence of molten LiCl/Ю and a small amount of iodine (see refs. 1, 2). a-YbPs forms bright black crystals of prismatic or columnar shape and is stable against dilute bases and non-oxidizing acids. a-YbPs is paramagnetic with μβίί
= 4.5 ць (see réf. 4).
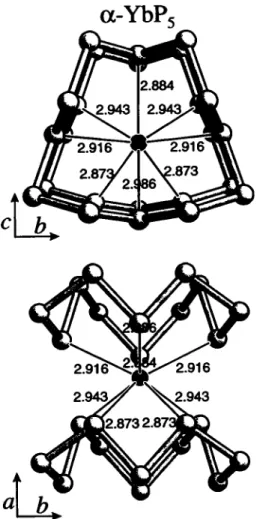
a-YbPs crystallizes in the space group P2\lm and is isotypic to NdPs (see ref. 4). The bond lengths vary between 2.161 A and 2.210 Â for the P - P bonds and between 2.873 Â and 2.986 Â for the Yb-P bonds.
PsYb, mœoclinic, P12i/wil (No. 11), a =4.873(1) К b =9.308(3) Â, с =5.287(1) Â, β =102.57(2)°, V=234.1 Â^ Z=2, ÄfF) =0.027, ÄwCFJ =0.030.
Table 1. Parameters used for the X-ray data coUectioD
Crystal:
Wavelength:
μ:
Diffiactometer:
Scan mode:
'^measuremeni·
2втах:
ЩЬк1)ип'цш·
Criterion for Io.
lHparam)refm:¡r·
Program:
black prism, metallic luster, size 0.5 X 0.1 X 0.5 mm Mo Ka radiation (0.71073 A) 214.9 cm"'_
SYNTEXPl ω
293 К 55°
641 /ο>2σ(/ο) 31
SHELXTL-plus
Table 2. Final atomic coordinates and displacement parameters (in Â^)
Atom Site X У ζ Un Un t/зз Un Un Un
Yb 2e 0.0043(1) 1/4 0.3494(1) 0.0213(3) 0.0205(3) 0.0209(3) 0 0.0030(1) 0
P(l) 4/ 0.3816(4) 0.5928(2) 0.0415(3) 0.0209(8) 0.0209(8) 0.0206(8) 0.0008(6) 0.0030(6) -0.0003(6) P(2) 4/ 0.2823(4) 0.5292(2) 0.4044(3) 0.0219(6) 0.0222(8) 0.0232(8) 0.0010(6) 0.0053(6) 0.0000(6)
P(3) 2e 0.2723(6) 1/4 0.8925(5) 0.021(1) 0.022(1) 0.024(1) 0 0.0039(9) 0
466
α - and β-Ytterbium pentaphosphide ß - Y b P <Y b 2
2. β - Y t t e r b i u m pentaphospЫde, ß - Y b P s
Source of material: ß-YbPs was formed as a byproduct of the synthesis of a - Y b P s (see refs. 1, 2). Crystals of a - and ß-YbPs c a n ' t be distinguished by their shape.
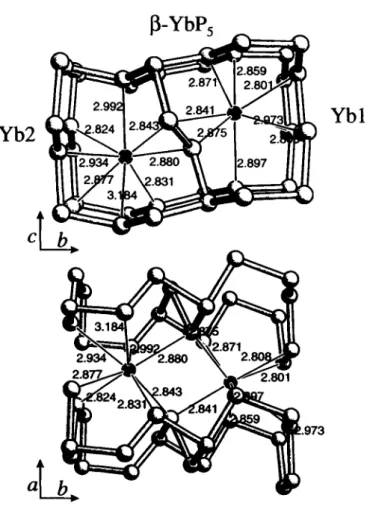
ß - Y b P s crystallizes in the space group P 2 | and forms a new Y b 1 structure type within the series of the rare earth pentaphosphides LnPs (Ln = La-Lu, except Eu) besides the LaPs and the NdPs structure type (see refs. 1 -3). Y b atoms and Ρ atoms form layers parallel (1(Ю). The polyanionic layers are more corrugated as in the a - m o d i f i c a t i o n , but f o r m also a net of condensed twelve membered rings (see lower part of the figure). The P - F distances vary between 1.840 Â and 2.230 Â. The Y b atoms are bound to 8 Ρ atoms with distances between 2.801 Â and 3.184 Â.
PsYb, monoclinic, P\2\ 1 (No. 4). a =5.249(1) Â, b =16.877(3) Â, с =5.317(1) Â, β =102.53(3)°, V = 4 5 9 . 8 Â ^ Z = 4 , ÄCF)=0.055, R ^ F ) =0.059.
Table 3. Parameters used for the X-ray data collection
:.973
Crystal: black prism, metallic luster.
size 0.5 X 0.1 xO.5 mm Wavelength: Mo Ka radiation (0.71073 Â)
μ: 209.9 cm"'
Diffractometer: SYNTEX PI
Scan mode: ω
measuremem'· 293 К
2втах: 55°
ЩИк1)ипщие·. 1218
Criterion for Io. / ο> 2 σ( / ο )
ìi(param)refined'· 108
I^ogram: SHELXTL-plus
Table 4. Final atomic coordinates and displacement parameters (in h?)
Atom Site X У ζ Un t/22 ί/зз и,2 Un U2}
Yb(l) 2a 0.0122(3) 0.0 0.0912(2) 0.0267(9) 0.0149(6) 0.0201(5) -0.0001(5) 0.0054(5) 0.0001(4) Yb(2) 2a 0.0146(3) 0.2610(1) 0.5943(2) 0.0225(8) 0.0156(6) 0.0214(5) 0.0001(5) 0.0005(5) 0.0005(4) P(l) 2a 0.773(2) 0.5039(5) 0.359(1) 0.028(4) 0.014(4) 0.023(3) 0.004(4) 0.005(3) -0.004(3) P(2) 2a 0.321(2) 0.2590(6) 0.139(1) 0.021(3) 0.017(3) 0.020(3) -0.005(4) 0.004(2) 0.005(3) P(3) 2a 0.699(2) 0.3563(5) 0.846(2) 0.018(4) 0.018(4) 0.024(3) 0.000(3) 0.007(3) 0.000(2) P(4) 2a 0.315(2) 0.4052(5) 0.659(2) 0.031(4) 0.017(4) 0.020(4) 0.002(4) 0.007(3) 0.002(3) P(5) 2a 0.405(2) 0.4374(4) 0.299(1) 0.030(4) 0.015(3) 0.013(3) -0.002(3) 0.009(3) -0.002(2) P(6) 2a 0.603(2) 0.3264(4) 0.218(2) 0.020(4) 0.015(3) 0.025(3) 0.001(3) 0.003(3) -0.003(3) P(7) 2a 0.375(2) 0.1264(4) 0.635(1) 0.025(3) 0.014(3) 0.020(3) 0.001(2) 0.006(3) 0.002(3) P(8) 2a 0.375(1) 0.1364(5) 0.224(1) 0.023(3) 0.015(2) 0.020(3) -0.001(2) 0.006(2) -0.002(3) P(9) 2a 0.791(1) 0.1334(4) 0.814(1) 0.020(3) 0.013(2) 0.021(3) -0.002(2) 0.004(2) 0.000(2) P(10) 2a 0.791(2) 0.1405(4) 0.230(1) 0.028(3) 0.018(3) 0.018(2) 0.001(2) 0.001(2) -0.001(2)
References
1. Wittmann, M.: Darstellung, Struktur und Eigenschaften von Polyphos- phiden und Polyarseniden der Seltenerdmetalle. Dissertation, Universität Münster, Germany 1977.
2. von Schnering, H. G.; Wichelhaus, W.; Wittmann, M.: New Polyphos- phides of the Rare Earth Metals. Abstracts Vth Intern. Conf. Solid Comp.
Trans. Elements, Uppsala, Sweden 1976.
Hartweg, M.: Über physikalische Eigenschaften der Seltenerd-Pentaphos- phide LnPs, von СеРг und LaAsz, sowie zur Kenntnis einiger neuer temärer Alkalimetall-Europium-Pnictide. Dissertation, Universität Stutt- gart, Germany 1987.
Wichelhaus, W.; von Schnering, H. G.: Die Pentaphosphide des Lanthans und Neodyms, LaPs und NdPs. Z. Anorg. AUg. Chem. 419 (1976) 77-86.
Sheldiick, G. M.: SHELXTL, an integrated system for solving, refining and displaying crystal structures from diffraction data. University of Göttingen, Germany 1978.