Curie Point Pyrolysis Gas Chromatography
Volltext
Abbildung
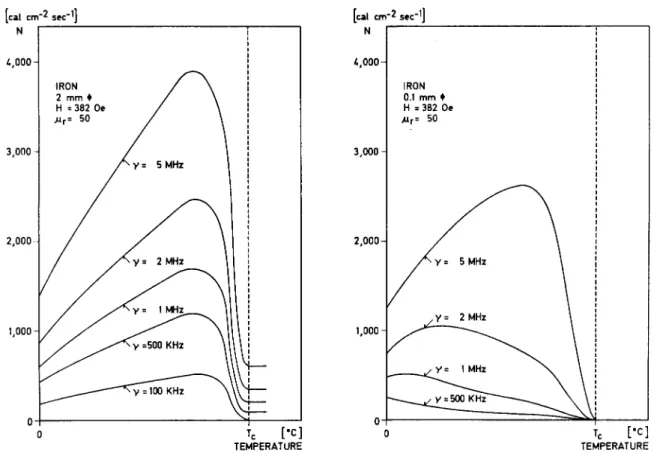
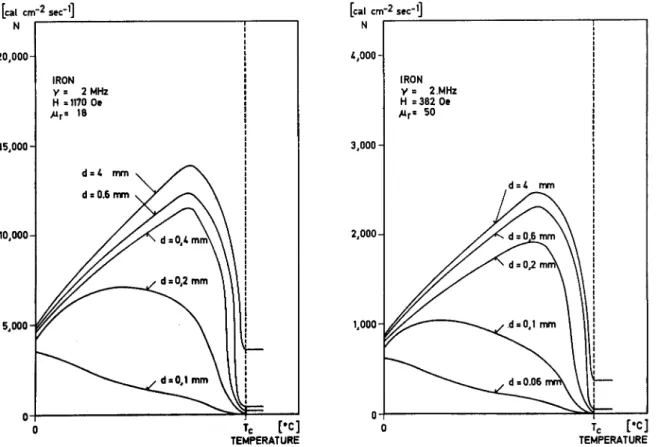
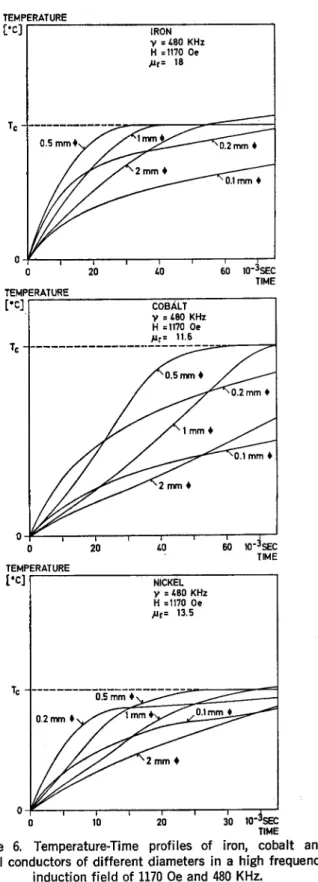
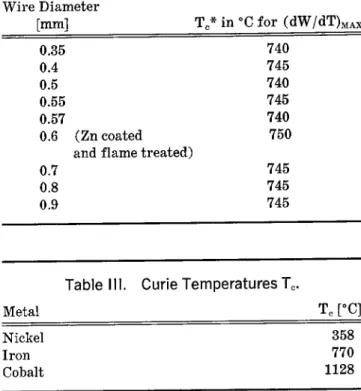
ÄHNLICHE DOKUMENTE
Storage temperature Tstg °C -55 +100 Stored as bare product after unpacking. Solder heat resistance of the
We also show that reversal readily occurs where conventionally, a Stoner-Wohlfarth type barrier (KV/ kBT) would ensure thermal stability on a long
Measurement of T 1 and T 2 relaxation times from different brain regions of anesthetized mice may be used for a correct quantification of metabolite concentrations in
The propagator of two-dimensional Dirac oscillator in the presence of a constant magnetic field is presented by means of path integrals, where the spin degree-of-freedom is described
The propagator of two-dimensional Dirac oscillator in the presence of a constant magnetic field is presented by means of path integrals, where the spin degree-of-freedom is described
However, mainly in astrophysics [2 – 4], space physics [5, 6], and plasma physics [7,8], there arises very often the problem of the motion of a charged particle when the intensity
The fractions of hexane and dichloromethane extraction from marupa´ (Simaruba amara) and (Bertholletia excelsa) leaves were analyzed by HT-HRGC (high temperature high resolu- tion
Alberto dos Santos Pereira a , Moˆnica Freiman de Souza Ramos b , Elisa Suzana Carneiro Poc¸as a , Patricia Castro Moreira Dias b , Elisabete Pereira dos Santos b , Joaquim