Research Collection
Doctoral Thesis
Studies on the prebiotic relevance of and symbioses between amyloidogenic peptides and vesicle-forming fatty acids
Author(s):
Bomba, Radoslaw Publication Date:
2020
Permanent Link:
https://doi.org/10.3929/ethz-b-000468367
Rights / License:
In Copyright - Non-Commercial Use Permitted
This page was generated automatically upon download from the ETH Zurich Research Collection. For more information please consult the Terms of use.
DISS. ETH NO. 27203
Studies on the prebiotic relevance of and symbioses between amyloidogenic peptides and vesicle-forming fatty acids
A thesis submitted to attain the degree of DOCTOR OF SCIENCES of ETH ZURICH
(Dr. sc. ETH Zurich) presented by RADOSŁAW BOMBA MSc, Lodz University of Technology
born on 30.07.1992 citizen of Poland
accepted on the recommendation of
Prof. Dr. Roland Riek, examiner Prof. Dr. Jeffrey W. Bode, co-examiner
Dr. Jason Greenwald, co-examiner
2020
Acknowledgements
I would like to express my gratefulness for being given an opportunity to conduct my doctoral studies in the department of physical chemistry at ETH Zürich. It was an amazing adventure that allowed me not only to gain a lot of experience and knowledge but also to a big extend shaped my life.
First of all, I would like to thank Prof. Dr. Roland Riek for giving me the opportunity to work in his research group. As the head, or as I would call him, the father of the group he is an excellent leader, who listens to everyone and always does his best to make sure that all the group members feel in his lab like home. His enormous knowledge and extraordinary ideas have been a great inspiration and encouragement for all the projects I took part in. His support in both, work and personal life were always of big importance to me.
I would like to thank Dr. Jason Greenwald and Dr. Witold Kwiatkowski for sharing with me their tremendous knowledge and experience. The discussions with both of them taught me how important it is to look at everything from different perspectives. All the advice they have given me was always accurate and very helpful. Their determination and extraordinary thinking helped me to significantly improve the quality of my work.
I would also like to thank Saroj Rout for sharing all his extensive expertise and all the interesting discussion embracing not only professional but also other topics.
Furthermore, I would like to thank Kristina Comiotto for all the support and help with any kind of concerns not necessarily associated with science as well as all the inspiring discussions.
Additionally, I would like to thank all the members of the group: Janina, Hari, Lukas, Juan, Natalia, Matthias, Pratibha, Dhiman, Cedric, Michael, Yanick, Felix, Piotr, Peter, Dean, Julien, Aditya, Sasha, Alois, Riccardo and all the others
who I forgot to mention. I would like to express my gratitude for all their help and for creating an amazingly friendly atmosphere in the lab. It has been an undeniable pleasure to be working with them.
As of last but not least I would like to thank my family and friends. I would especially like to thank my parents Elżbieta and Marek. Their love, support, and all the good values they have instilled in me enabled me to achieve everything what I have achieved so far.
Abstract
There are millions of different species of living organisms in the ecosystem of Earth, all of them enormously complex machineries controlled through an intricate network of chemical and physical processes. Even the simplest living organisms exhibit an extraordinarily high molecular complexity that leaves organic chemists scratching their heads. Therefore, it is natural to ask how these complicated entities arose from early Earth prebiotic environment and which molecules and processes were essential. There are many different hypotheses emphasizing the roles of different kinds of biomolecules like RNA, peptides or lipids in life’s origins. One of the hypotheses proposes that peptide amyloids, highly ordered, β-sheet-rich peptides assemblies that can be considered as primitive analogues of proteins, played an early role. Their resistance to harsh conditions could allow amyloids to evolve and increase in their complexity in an early Earth environment.
Following this path, the first part of this thesis focuses on the templating properties of amyloids in peptide elongation reactions that utilize carbonyl sulfide as a plausibly prebiotic activating agent. The influence of the amyloids on the stereo-, regio- and sequence selectivity of amino acid addition to a substrate peptide has been investigated with the results demonstrating a significant enhancement of the selectivity for systems in which peptides are incorporated in amyloid assemblies. Additionally, the peptides in the amyloid co-aggregates have been found to be protected from some of the side reactions that occur with the carbonyl sulfide-induced peptide bond formation.
My further investigations on the properties of the amyloids and amyloidogenic peptides in the context of life’s origins were motivated by the fact that there are many reports showing interactions between disease-associated amyloids and biological membranes. It has been hypothesized that the presence of such interactions is due to an inherent affinity that arises between these repetitive assemblies through avidity. Therefore, my interest in the molecular origins of
life have led me to investigate the nature of such interactions in the context of their molecular predecessors.
In the second part of the thesis the emphasis has been placed on the interactions between prebiotic amyloidogenic peptides and vesicle-forming fatty acids. The results have shown that an amyloidogenic peptide which forms amyloids at pH 11 but remains soluble at neutral pH due to its positive electrostatic character, in mixture with fatty acids is able to form novel co-aggregates, whose structures are sensitive to both the fatty acid concentration and identity. The further analysis of decanoic acid with a panel of 13 other basic amyloidogenic peptides confirmed the general nature of the observed interactions. Moreover, these interactions have been shown to be mutually beneficial, expanding the phase space of both peptides and fatty acids.
Moreover, the investigation of amino acid polymerization in the presence of amphiphile assemblies indicate that vesicles of simple fatty acids and lipids can support the spontaneous internal formation of amyloids. The results show that fatty acid or lipid vesicles can act both as a filter, allowing the selective passage of activated amino acids, and as a barrier, blocking the diffusion of the amyloidogenic peptides that form spontaneously inside the vesicles. Thus under conditions of continuous dilution or in the presence of a competing external reaction, the inside of the vesicle offers a haven for the accumulation of amyloidogenic peptides that would otherwise be washed away or never formed due to competing reactions.
Zusammenfassung
Im Ökosystem der Erde gibt es Millionen verschiedener Arten lebender Organismen, allesamt ungeheuer komplexe Maschinen, die durch ein kompliziertes Netzwerk chemischer und physikalischer Prozesse reguliert werden. Sogar die einfachsten lebenden Organismen zeigen eine außergewöhnlich hohe molekulare Komplexität, die den organischen Chemikern ein Kopfzerbrechen bereitet. Daher liegt es nahe zu fragen, wie diese komplizierten Gebilde aus der frühen präbiotischen Umwelt der Erde entstanden sind und welche Moleküle und Prozesse wesentlich waren. Es gibt viele verschiedene Hypothesen, die die Rolle der verschiedenen Arten von Biomolekülen wie RNA, Peptide oder Lipide bei der Entstehung des Lebens betonen. Eine der Hypothesen besagt, dass Peptidamyloide, hoch geordnete, β- blattreiche Peptidanordnungen, die als primitive Analoga von Proteinen betrachtet werden können, eine frühe Rolle spielten. Ihre Widerstandsfähigkeit gegen harte Bedingungen könnte es den Amyloiden ermöglichen, sich in einer frühen Erdumgebung zu entwickeln und ihre Komplexität zu erhöhen.
Diesem Weg folgend, konzentriert sich der erste Teil dieser Arbeit auf die Vorlage-Eigenschaften von Amyloiden in Peptidverlängerungsreaktionen, die Carbonylsulfid als möglicherweises präbiotisches Aktivierungsmittel nutzen.
Der Einfluss der Amyloide auf die Stereo-, Regio- und Sequenzselektivität der Aminosäurenaddition an ein Substratpeptid wurde untersucht, wobei die Ergebnisse eine signifikante Steigerung der Selektivität für Systeme zeigen, in denen Peptide in Amyloidanordnungen eingebaut sind. Zusätzlich wurde es festgestellt, dass die Peptide in den Amyloid-Koaggregaten vor einigen der Nebenreaktionen geschützt werden, die bei der durch Carbonylsulfid induzierten Bildung von Peptidbindungen auftreten.
Unsere weiteren Untersuchungen zu den Eigenschaften der Amyloide und amyloidogenen Peptide im Zusammenhang mit dem Ursprung des Lebens wurden dadurch motiviert, dass es viele Berichte gibt, die Wechselwirkungen
zwischen krankheitsassoziierten Amyloiden und biologischen Membranen zeigen. Es wurde die Hypothese aufgestellt, dass das Auftreten solcher Interaktionen auf eine inhärente Affinität zurückzuführen ist, die zwischen diesen sich wiederholenden Aggregaten durch Avidität entsteht. Deshalb hat mich mein Interesse an den molekularen Ursprüngen des Lebens dazu veranlasst, die Natur solcher Wechselwirkungen im Kontext ihrer molekularen Vorgänger zu untersuchen.
Im zweiten Teil der Arbeit wurde der Schwerpunkt auf die Wechselwirkungen zwischen präbiotischen amyloidogenen Peptiden und vesikelbildenden Fettsäuren gelegt. Die Ergebnisse haben gezeigt, dass ein amyloidogenes Peptid, das bei pH 11 Amyloide bildet, aber aufgrund seines positiven elektrostatischen Charakters bei neutralem pH-Wert löslich bleibt, in Mischung mit Fettsäuren in der Lage ist, neuartige Koaggregate zu bilden, deren Strukturen sowohl auf die Fettsäurekonzentration als auch auf die Identität der Fettsäuren empfindlich reagieren. Die weitere Analyse von Decansäure mit einem Panel von 13 anderen basischen amyloidogenen Peptiden bestätigte die allgemeine Natur der beobachteten Wechselwirkungen. Außerdem erwiesen sich diese Interaktionen als gegenseitig vorteilhaft, da sie den Phasenraum sowohl der Peptide als auch der Fettsäuren erweitern.
Darüber hinaus weist die Untersuchung der Aminosäurepolymerisation in Gegenwart von amphiphilen Assemblierungen darauf hin, dass Vesikel aus einfachen Fettsäuren und Lipiden die spontane interne Bildung von Amyloiden unterstützen können. Die Ergebnisse zeigen, dass Fettsäure- oder Lipidvesikel sowohl als Filter funktionieren können, der die selektive Durchleitung aktivierter Aminosäuren erlaubt, als auch als Barriere, die die Diffusion der amyloidogenen Peptide blockiert, die sich spontan im Inneren der Vesikel bilden. Unter Bedingungen kontinuierlicher Verdünnung oder in Gegenwart einer konkurrierenden äusseren Reaktion bietet das Innere des Vesikels somit einen Zufluchtsort für die Anhäufung von amyloidogenen Peptiden, die ansonsten weggespült oder aufgrund von konkurrierenden Reaktionen nie gebildet würden.
Contents
Acknowledgements ... 3
Abstract ... 5
Zusammenfassung ... 7
1. Introduction ... 13
1.1. Amyloids in prebiotic environment ... 14
1.1.1. Amino acids and peptides in the Earth’s early atmosphere ... 14
1.1.2. Amyloid structure and its characterization ... 18
1.1.3. Amyloids in origins of life ... 21
1.2. Fatty acids as prebiotic precursors of modern lipids ... 25
1.2.1. Amphiphiles ... 25
1.2.2. Micelles and vesicles of fatty acids ... 27
1.2.3. Compartmentalization in origins of life ... 31
2. Carbonyl sulfide as a prebiotic activation agent for stereo- and sequence-selective, amyloid-templated peptide elongation ... 33
2.1. Abstract ... 33
2.2. Introduction ... 34
2.3. Results and discussion ... 35
2.3.1. Stereoselective addition of amino acids to R(FR)3 templated by (FE)4 ... 35
2.3.2. Sequence-selective addition of amino acids to the R(FR)3/(FE)4 amyloid ... 44
2.4. Conclusions ... 46
3. Cooperative induction of ordered peptide and fatty acid
aggregates ... 48
3.1. Abstract ... 48
3.2. Introduction ... 49
3.3. Results and Discussion ... 51
3.3.1. Amyloidogenic peptide and fatty acid characterizations ... 51
3.3.2. Cooperative interactions between (OV)4 and fatty acids ... 53
3.3.3. Structural characterization of peptide-fatty acid co-aggregates ... 62
3.3.4. General nature of cooperative interactions between amyloidogenic peptides and fatty acids ... 72
3.3.5. Mutualism in peptide fatty acid interactions ... 75
3.4. Conclusions ... 77
4. Prebiotic peptide synthesis and spontaneous amyloid formation inside a proto-cellular compartment ... 79
4.1. Abstract ... 79
4.2. Introduction ... 80
4.3. Results and Discussion ... 83
4.3.1. Amino acids display a large range of membrane permeability ... 83
4.3.2. Externally activated amino acids condense with peptides inside of vesicles ... 87
4.3.3. Vesicle-dependent amyloid of Vn(DV)4 forms upon addition of Val to encapsulated V(DV)4 ... 90
4.3.4. Membrane selectivity provides haven in chemically unfavorable conditions and permits intra-vesicular amyloid under non-diluting conditions ... 96
4.3.5. De novo peptides form amyloids inside “empty” vesicles ... 98
4.4. Conclusions ... 102
5. Conclusion and Outlook ... 103
6. Materials and Methods ... 105
6.1. Carbonyl sulfide as a prebiotic activation agent for stereo- and sequence-selective, amyloid-templated peptide elongation. ... 105
6.1.1. Reagent preparation and purity ... 105
6.1.2. Amyloid formation ... 106
6.1.3. Amino-acid addition reactions and analyses ... 106
6.2. Cooperative induction of ordered peptide and fatty acid aggregates 107 6.2.1. Materials ... 107
6.2.2. Fatty acid solutions ... 108
6.2.3. Amyloid formation ... 108
6.2.4. Formation of peptide-fatty acid mixtures ... 109
6.2.5. Circular dichroism (CD) ... 109
6.2.6. Critical micelle and critical vesicle concentrations ... 109
6.2.7. Fluorescence anisotropy ... 118
6.2.8. Cryo-EM and negative stain TEM ... 118
6.2.9. Attenuated total reflectance Fourier transform infrared spectroscopy (ATR-FTIR) ... 119
6.2.10. X-ray diffraction ... 119
6.2.11. Small-Angle X-ray Scattering (Rigaku) ... 119
6.2.12. HPLC analysis ... 121
6.2.13. Protease sensitivity assay ... 122
6.3. Prebiotic peptide synthesis and spontaneous amyloid formation inside a proto-cellular compartment ... 122
6.3.1. Vesicle preparation ... 122
6.3.2. Vesicle permeation monitored by NMR ... 123
6.3.3. HPLC-MS analysis ... 124
6.3.4. CDI-activation ... 125
6.3.5. Amino acid addition reactions inside of vesicles ... 125
6.3.6. Matrix-assisted laser desorption/ionization Fourier transform ion cyclotron resonance mass spectrometry (MALDI FT-ICR MS) ... 126
6.3.7. Circular dichroism (CD) spectroscopy ... 127
6.3.8. Cryo-EM ... 127
Appendix ... 129 Bibliography ... 145
1. Introduction
What is the origin of life? What kind of processes and molecules were involved in abiogenesis? What were the first species, which might be considered as living organisms? While the development of science and technology is progressing rapidly, these basic and deceptively simple questions remain still unsolved. The mystery of transition from complex prebiotic soup into the first biological system seems to be the most difficult to solve. There is very little information about the environment at the time when the life supposedly arose between 3.5 (Noffke et al. 2013) and 4.3 billion (Cavosie et al. 2005) years ago. The limited observations of preserved structures (Schopf 2006; Schopf et al. 2007; Bell et al. 2015) and analysis of rocks (Ohtomo et al. 2014) and asteroids (Meier et al.
1998; Hsieh and Jewitt 2006) are the main sources of information. Lack of insights on the early life environment left an open field for a lot of competing and seemingly exclusive hypotheses such as replication first (Orgel 1968), metabolism first (Wächtershäuser 1988), and compartmentalization first (Segré et al. 2001) or RNA world (Gilbert 1986), peptide world (Rode 1999; Oba et al.
2005), and amyloid world (Carny and Gazit 2005; Maury 2008, 2009;
Greenwald and Riek 2010, 2012). It is evident that these hypotheses tend to oversimplify the problem and that at one point in the origin or evolution of early life, the primary molecules of biology, namely nucleic acids, amino acids, aliphatic compounds and carbohydrates became intimately intertwined. The emergence of these distinct molecular systems could have been sequential or separated in space or all at once as it has been postulated recently (Szostak 2011;
Patel et al. 2015).
This thesis focuses on two such primary molecules: peptides and their highly ordered structures, namely amyloids, as well as vesicle-forming fatty acids.
These species, whose relevance in the prebiotic evolution towards life is an underlying theme of my research, are introduced in the following sections.
1.1. Amyloids in prebiotic environment
1.1.1. Amino acids and peptides in the Earth’s early atmosphere
The chemical composition of the atmosphere on Earth in its early stage is presumed to play an important role in the origin of life. The famous Miller-Urey experiment from the 1950s showed that amino acids and other prebiotically relevant molecules could have been formed in the Earth’s early atmosphere (Miller 1953, 1955; Miller and Urey 1959; Oró and Kamat 1961; Johnson et al.
2008). The experiment was designed to mimic the chemistry that would have been the result of electrical discharges or UV radiation absorbed in reduced atmosphere composed mostly of methane, ammonia and water. The molecules detected by Miller in the original experiment are shown in Fig. 1.1.
Figure 1.1. Molecules detected in the first Miller-Urey type experiment.
OH
O Acetic acid H2N
OH
O Alanine H2N
OH
O Glycine
OH
O NH2
-Aminobutyric acid
OH
O H2N
-Alanine
OH
O Formic acid
Glycolic acid Lactic acid
OH
O Propionic acid
NH
OH
O Sarcosine
HO
OH
O
OH
O OH
A re-analysis of Miller’s samples in 2008 with more sensitive instrumentation expanded the list of detected products to include more of the proteinogenic amino acids i.e. valine, serine, aspartic acid, glutamic acid and phenylalanine (Johnson et al. 2008).
Furthermore, it has been shown that small organic molecules could be brought to the Earth by meteorites like Murchison meteorite (Wolman et al. 1972;
Thompson et al. 1987; Pierazzo and Chyba 1999; Schmitt-Kopplin et al. 2010) or formed in hydrothermal processes (Hennet et al. 1992; Huber and Wächtershäuser 1997, 2006; Johnson et al. 2008; Martin et al. 2008; Roldan et al. 2015).
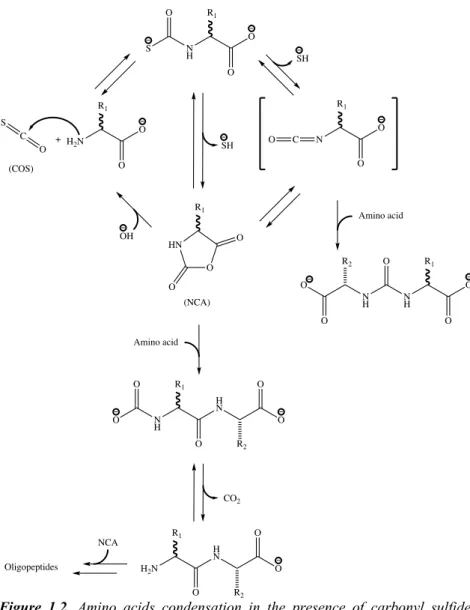
The formation of polymers of amino acids, namely peptides, was also shown to be possible in the prebiotic environment. One of the ways is the condensation in the presence of the volcanic gas carbonyl sulfide (COS) (Leman et al. 2004).
The reaction of COS with amino acids produces a thiocarbamate, which is further transformed into an N-carboxyanhydride (NCA), a highly reactive species. The carbonyl group of the NCA is then attacked by the amine group of amino acid resulting in a peptide bond formation (Fig. 1.2).
Figure 1.2. Amino acids condensation in the presence of carbonyl sulfide.
(Adapted from: Leman L, Orgel L, Ghadiri MR (2004) Carbonyl Sulfide- Mediated Prebiotic Formation of Peptides. Science 306:283–286)
S N
H
O R1
O
O
H2N R1
O
O S
C O +
SH N
R1 O
O C
O SH
OH HN
O R1
O
O
Amino acid
Amino acid
NH N
H
O R1
O
O R2
O
O
NH R1
HN
O R2
O O
O O
CO2
H2N R1
HN
O R2
O O (COS)
(NCA)
NCA Oligopeptides
Other possibilities by which amino acids can be condensed into peptides include copper catalysis in high salt concentration and high temperature (Rode 1999), reaction driven by CO in the presence of coprecipitated (Fe,Ni)S and Na2S (Huber et al. 2003), and dehydration-hydration cycles (Fig. 1.3) (Rodriguez- Garcia et al. 2015). Based on those results it is reasonable to believe that the simple peptides could be present in the early Earth environment.
Figure 1.3. Peptide bond formation due to dehydration-hydration cycles.
(Adapted from: Rodriguez-Garcia M, Surman AJ, Cooper GJT, et al (2015) Formation of oligopeptides in high yield under simple programmable conditions. Nat Commun 6:1–7)
H2N
OH O
R1
AA
H2O
HN
OH O
R1 O H2N
R2 AA
H2O
AA
H2O
HN
OH O
R1 O NH
R2 O H2N
R3 NH
HN
O
O R1
R2
H2O
AA
HN O
R n
Polymer Trimer
Cyclic dimer
Dimer
Amino acid monomer (AA)
1.1.2. Amyloid structure and its characterization
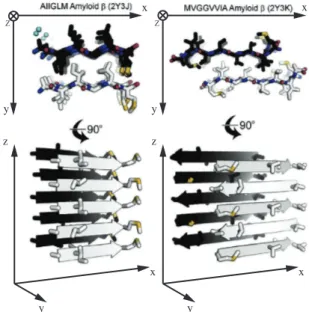
Amyloids are the aggregates of β-sheet structured peptides or proteins assembled further into stable layered assemblies that are characterized by a fibrillar morphology. Fig. 1.4 shows two examples of the amyloid structures. In the figure, the fibers are oriented such that the axis of the fiber is parallel to the z axis with the extended β-sheets laying in the xz plane.
Figure 1.4. Examples of the amyloid structures of two different peptides from two different perspectives. The left structure is comprised of parallel and the right one of antiparallel β-sheets. The sequences and PDB IDs of the peptides are given above the structures. (Adapted from: Riek R, Eisenberg DS (2016) The activities of amyloids from a structural perspective. Nature 539:227–235) The β-strands in amyloids are arranged perpendicularly to the axis of the fiber.
Such a structure is also known as a cross-β structure (Sunde et al. 1997). The name cross-β refers to the two sets of X-ray diffraction lines orthogonal to each
z
y
x z
y
x y
x
y
x
Z Z
other (Nilsson 2004; Fändrich 2007; Riek and Eisenberg 2016) that arise from aligned amyloid samples. As shown in Fig. 1.5 one set corresponds to the interstrand spacing while the other one to the intersheet spacing.
Figure 1.5. Scheme showing the diffraction pattern created by the amyloid. The example fiber is characterized by meridional reflection at 4.8 Å and equatorial reflection at 10 Å. (Source: Riek R, Eisenberg DS (2016) The activities of amyloids from a structural perspective. Nature 539:227–235)
Even though the structure of the amyloid can be characterized by X-ray diffraction, there are more methods useful in its characterization. The most common methods are electron microscopy (EM) providing the information about morphology of the fibrils, circular dichroism spectroscopy (CD) allowing the determination of the secondary structure composition of the peptide/protein (Kelly et al. 2005) and infrared spectroscopy (IR) (Susi et al. 1967; Timasheff et al. 1967; Krimm and Bandekar 1986; Jackson and Mantsch 1991; Oberg et al.
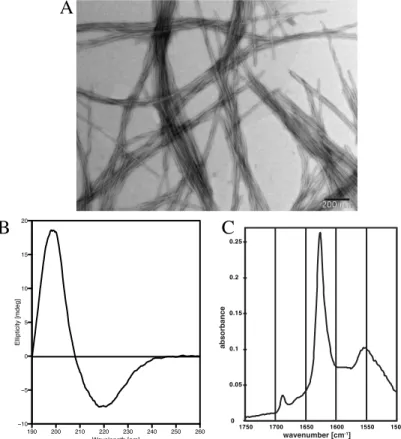
1994; Fink 1998), which in addition to providing secondary structural information can give more detailed insight into the geometry of the β-sheets (parallel or antiparallel). Examples of the electron micrograph, CD spectra and IR spectra of an amyloid are shown in Fig. 1.6. The EM image shows the bundles of fibrils formed by the peptide. The CD spectra is characterized by the minimum at around 217 nm and a maximum at around 195 nm, which is indicative of β-sheet folding (Greenfield and Fasman 1969). In the ATR-FTIR
spectrum the two absorbance peaks can be found, one with maximum at around 1625 cm-1 and the second one at around 1685 cm-1. The first peak corresponds to the amide I band characteristic for the β-strand (Zandomeneghi et al. 2004), while the second peak is indicative of antiparallel geometry of the β-sheet (Toniolo and Palumbo 1977; Krimm and Bandekar 1986).
Figure 1.6. In panel A the electron micrograph of DFRFRF-NH2 amyloids is shown. Panels B and C represent CD spectrum of Ac-VDVDVDVDV-NH2 and ATR-FTIR spectrum of poly-valine fibrils respectively. (Source of panel C:
Greenwald J, Friedmann MP, Riek R (2016) Amyloid Aggregates Arise from Amino Acid Condensations under Prebiotic Conditions. Angew Chem Int Ed 55:11609–11613)
0 0.05 0.1 0.15 0.2 0.25
1500 1550 1600 1650 1700 1750
wavenumber [cm-1]
absorbance
190 200 210 220 230 240 250 260
0 5 10 15 20
Wavelength [nm]
Ellipticity [mdeg]
A
B C
1.1.3. Amyloids in origins of life
Amyloids as highly ordered and stable structures show a high level of robustness. They are resistant to the extreme conditions of temperature and pH (Guijarro et al. 1998; Chiti et al. 1999; Polverino de Laureto et al. 2003; Marcon et al. 2005), which could have been present in the early Earth environment.
Moreover, peptides incorporated in such aggregates show stability and resistance to degradation (Brack and Spach 1980; Bonner et al. 1981; Toledano et al. 2006; Greenwald et al. 2018). The high stability of the amyloids could have allowed them to exist long enough to evolve in harsh conditions. Therefore, it is possible that amyloids played an important role in the origins of life.
It was shown that amyloids can be spontaneously formed by the polymerization products of the proteinogenic amino acids valine and alanine as well as of the mixture of glycine, alanine, aspartate and valine performed in the presence of COS gas (Greenwald et al. 2016). Furthermore, the insoluble fraction after polymerization of the mixture of amino acids was found to have mostly hydrophobic amino acids valine and alanine, showing that the amyloid formation is selective regarding the amino acid composition.
Typically, amyloids consist of the β-strands of at least 6 residues in length.
Nevertheless, there are reports showing even shorter peptides like tetrapeptides NNQQ (Sawaya et al. 2007), KFFE and KVVE (Tjernberg et al. 2002) or tripeptides GYE (Naskar et al. 2008) being able to assemble into amyloid structures. Even though those peptides are less prebiotically relevant, they could have evolved in the later stage of the abiogenesis.
Another important aspect in which the amyloids could have a significant contribution is the origin of homochirality. Pauling and Corey defined in their model in 1951 the stereochemical requirement for the β-strand in a β-sheet to be isotactic. This requirement was later confirmed by Brack and Spach in 1979 (Brack and Spach 1979, 1980). Their statistical model, which was supported by Hitz and Luisi (Hitz and Luisi 2004) showed that the β-sheets formed from the peptides having random D and L residues in the sequence were enriched with L
residues. The formation of amyloids as an assembly of β-sheets would have in this way selected for the isotactic or nearly isotactic peptides and also protect them from the degradation, providing a tool for the chiral amplification.
The majority of chemical reactions performed by living organisms are energetically unfavorable and would be impossible to perform without special machineries, typically protein catalysts. Those highly organized and complex structures evolved over billions of years to provide the optimal conditions for the processes essential for life. It is reasonable to assume based on their simplicity and similarity that peptides are the prebiotic precursors of proteins.
Numerous lines of research have tried to optimize and understand the catalytic activities of peptides often in an effort to replace complicated and expensive enzymes in industrial processes. However, since short peptides cannot provide a well-defined active site, they are much more limited then proteins. Still, there is evidence of the larger assemblies of peptides and their analogues showing enzyme-like catalytic activities (Zozulia et al. 2018). The highly ordered, β- sheet-rich amyloids could be considered as good analogues of proteins, some of which also contain a high degree of β-sheets in their structures, like for example archaeal carbonic anhydrase (Iverson et al. 2000) or tachylectin (Beisel et al.
1999). It has been demonstrated that peptide amyloids can catalyze simple reactions like hydrolysis and oxidation, but also retro-aldol reactions or stereoselective aldol condensations (Barbier and Brack 1988, 1992; Pérez‐Payá et al. 1996; Rufo et al. 2014; Zhang et al. 2014; Friedmann et al. 2015;
Makhlynets et al. 2016; Tena-Solsona et al. 2016; Monasterio et al. 2017;
Omosun et al. 2017; Lengyel et al. 2018).
The highly ordered and repetitive structure of peptide amyloids makes them a promising molecular platform on which to build a self-replicating system.
Peptides that are incorporated in the amyloid can act as a template for the synthesis of the complementary polymers from shorter building blocks. Fig. 1.7 shows a scheme of templated ligation of two short peptides that each comprise one half of the longer polymer (Takahashi and Mihara 2004; Rubinov et al.
2009). The presence of the template improves the rate of the condensation and increases the yield of the product.
Figure 1.7. Schematic showing the templated ligation of two substrate peptide analogues each forming one half of the template peptides that are incorporated in amyloid structures. The substrate peptides are ABA-EFEFA-COSR and CEFEFEP-CONH2. ABA stands for 4-acetamidobenzoate. (Source: Rubinov B, Wagner N, Rapaport H, Ashkenasy G (2009) Self-Replicating Amphiphilic β- Sheet Peptides. Angew Chem Int Ed 48:6683–6686)
Similarly to the work of Rubinov et al., another example utilizes the peptides with alternating sequences of hydrophilic and hydrophobic amino-acids designed the way that the substrate and the template can form a β-sheets based on the charge interactions, which can further aggregate into the cross-β fibers (Rout et al. 2018). In this system, the substrate is just a single peptide that is shorter than the template allowing the catalytic pockets to be formed. Presence of such pockets allow the system to template the addition of single amino acids to the substrate. A schematic showing the designed system is depicted in Fig. 1.8.
Figure 1.8. A model of amyloid templated amino acid addition. The circles indicate the hydrophilic residues with negative (red) and positive charge (blue).
Hydrophobic amino acids are depicted as grey squares. Green blurred circles show the pockets acting as active sites. (Adapted from: Rout SK, Friedmann MP, Riek R, Greenwald J (2018) A prebiotic template-directed peptide synthesis based on amyloids. Nat Commun 9:1–8)
+
The system presented in Fig.1.8 demonstrates a significant enhancement of stereoselectivity. The generalizability of the system was demonstrated with several other substrate/template pairs including a substrate with side chain amines that exhibited regioselective amino acid addition in the presence of its template.
Resistance to the harsh conditions and increased stability of its components allows amyloids to evolve and increase in their complexity. Its highly ordered structure makes it a potentially good tool for chiral amplification and templated reactions. Additionally, the repetitive structure of amyloids opens the possibility for the interactions with other prebiotically relevant species. Taken all together, amyloids are good candidates to have been involved in prebiotic processes and can be presumed to have played an important role in the origins of life.
1.2. Fatty acids as prebiotic precursors of modern lipids
1.2.1. Amphiphiles
Amphiphiles are the molecules possessing both, hydrophobic and hydrophilic properties. The biological amphiphiles typically have a longer hydrophobic hydrocarbon moiety with a smaller hydrophilic group which can be categorized depending on the charge into anionic, cationic, zwitterionic or polar uncharged.
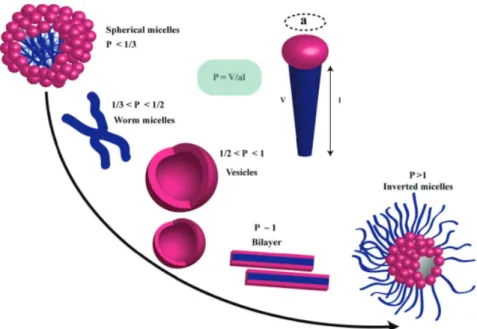
The most common examples of biological amphiphiles are lipids, cholesterol, fatty acids, and fatty alcohols. Typically, amphipathic molecules containing one hydrophobic chain behave as surfactants (surface active agents) while the double-chain amphiphiles are called lipids and are the building blocks of biological membranes. The surfactant-like amphiphiles often have very simple structures yet can form complex and highly repetitive assemblies. Due to their hydrophobic/hydrophilic double nature, their behavior depends not only on their atomic structure but also on the composition of solvent, their concentration, temperature and in many cases also on pH. Therefore, they are subject of many studies trying to investigate their properties and utilize them for designing new materials, drug delivery systems, bioreactors, cosmetics or for studying the properties of membrane proteins. The basic parameter that can be used to describe the preferred aggregation state or phase into which the surfactant can assemble is the packing parameter (Israelachvili et al. 1976, 1980; Nagarajan and Ruckenstein 1991; Nagarajan 2002). Fig. 1.9 depicts how the preferred aggregation state changes over different values of the packing parameter, which is calculated as the ratio of the molecular volume (V) to the product of effective maximum chain extension (l) and optimum headgroup area (a) (Kulkarni et al.
2011).
Figure 1.9. The characteristic aggregation states of a typical surfactant, the stability of each depends on the packing parameter. (Source: Dhasaiyan P, Prasad BLV (2017) Self-Assembly of Bolaamphiphilic Molecules. Chem Rec 17:597–610)
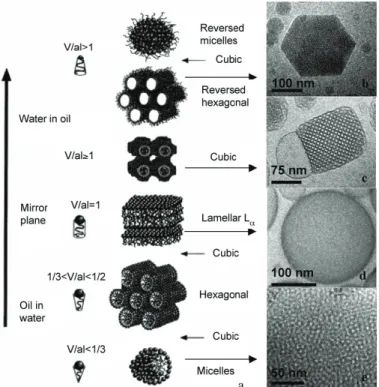
It is important to keep in mind that the packing parameter and therefore, the structure of the assembly, can change depending on the temperature or the solvent conditions. Fig. 1.10 presents more of the possible structures that can be adapted by the amphiphiles in water while changing the oil to water ratio.
Thus, despite their very simple chemical structures, amphiphiles can display very complex behavior, being able to form numerous different aggregate phases depending on the environmental conditions. The capacity of forming a localized hydrophobic environment as well as forming semipermeable membranes are the main features that are potentially advantageous in prebiotic environment. The fatty acids and their derivatives are some of the simplest and most prebiotically plausible amphiphiles and therefore next part of this chapter will focus on the properties of their micelles and vesicles.
Figure 1.10. Assemblies formed by the amphiphiles in different water to oil ratios. (Source: Sagalowicz L, Leser ME, Watzke HJ, Michel M (2006) Monoglyceride self-assembly structures as delivery vehicles. Trends Food Sci Technol 17:204–214)
1.2.2. Micelles and vesicles of fatty acids
Micelles are dynamic aggregates of amphiphiles with fluid hydrophobic interior.
Increasing the concentration of the surfactant in water causes initially the saturation of the water-air interface and as a result the decrease of the surface tension (Watanabe et al. 2005). The reason for that is the unfavorable interaction between polar water molecules and hydrophobic tails of surfactant. At high enough concentration the saturation on the surface is reached and the micelles
start to form (Fig. 1.11). This concentration is called critical micelle concentration (CMC) and it is a characteristic value for each surfactant under a given condition of temperature, ionic strength, pH etc. The image of the micelle in the Fig. 1.11 is simplified and does not reflect its real structure. Studies have shown that micelles have a fluid interior with the hydrophobic tails being randomly distributed and headgroups moving at near diffusion-controlled rate (Israelachvili et al. 1977; Menger 1979; Jorge 2008).
Figure 1.11. Distribution of surfactant molecules in aqueous solution above its CMC. (Source: Romsted LS (2012) Introduction to Surfactant Self-Assembly. In:
Supramolecular Chemistry. American Cancer Society)
After the micelles, the second most common assembly of amphiphiles in aqueous solutions are vesicles. A vesicle is composed of a self-closed curved bilayer of amphiphiles with hydrophilic head groups oriented towards water and hydrophobic tails forming the essentially water-free layer that delineates the inside from the outside of the vesicle (Fig. 1.12). In analogy to the CMC value, the concentration of amphiphile at which the vesicles start to form is called the critical vesicle concentration (CVC) or critical bilayer concentration (CBC) which is dependent mostly on the type of amphipathic compound but also on the other parameters like temperature, pressure, pH and solvent composition.
Similarly to micelles, vesicles of single chain amphiphiles are dynamic systems being always in rapid equilibrium with micelles and non-associated amphiphiles.
Figure 1.12. Schematic structure of a lipid vesicle. (Source: Furukawa, Kazuaki (2009) Artificial Cell Membrane on Patterned Surface-Growth Control and Microchannel Device Application)
Vesicles are relatively complex structures being able to adapt various shapes and sizes. The sizes of vesicles vary from tens of nanometers to hundreds of microns.
The typical shape of the vesicle in equilibrium is spherical, but due to disruptions from external sources it can be significantly changed. The morphology of the vesicles can also vary due to multilamellarity (several vesicles encapsulated inside of each other in a centrosymmetrical manner) or multivesicularity (several small vesicles encapsulated in the larger one) (Walde et al. 2014). The size and morphology of the vesicles can be relatively well modulated by different preparation methods (Walde and Ichikawa 2001).
With respect to micelle and vesicle formation it has to be kept in mind that not all amphiphiles are capable of forming both types of assemblies. One of the main factors determining the ability to form a given structure is the packing parameter.
Since for the typical surfactants this value is <1/3 they form mostly micellar assemblies, while lipids with their double-chain have values in the range of 1/2 to 1 and therefore preferentially form bilayers and vesicles.
However, some fatty acids, which are typically considered as surfactants have the ability to form both micelles and vesicles (Gebicki and Hicks 1973;
Hargreaves and Deamer 1978). This phenomenon can be easily explained by the fact that depending on the pH of the solution, fatty acids can carry a charge, remain neutral and the ratio of these two forms in solution will depend primarily
Hydrophilic Hydrophobic
on the pH. This property of fatty acids as amphiphiles make it possible to alter their packing parameter by adjusting the pH of the solution. Above the CMC value and at high pH fatty acids can only form micelles due to repulsive interactions between negatively charged headgroups (large optimal area of the head group is decreasing the value of the packing parameter). On the other extreme, at low pH all the fatty acid molecules are protonated and therefore lack a repulsive interaction between the polar region of the molecule. Also, the loss of favorable interactions with aqueous surrounding causes them to form aggregates or a liquid/liquid (oil-like) phase separation. At a pH close to the pKa
of the acid, there will be coexisting charged and uncharged species allowing the carboxylic groups create hydrogen bonds thus reducing the average area of the headgroup and increasing the value of the packing parameter. As a result, at the correct pH it is possible for the fatty acids to form bilayers (Rosano et al. 1969;
Haines 1983; Cistola et al. 1988).
1.2.3. Compartmentalization in origins of life
There are several hypotheses on how fatty acid vesicles could have been formed in the early Earth environment. One of the possible scenarios is the formation during hydration and dehydration of the thermal pools (Fig. 1.13). During this process the multilamellar films of amphiphiles accumulated at the bottom of the pools while drying could form vesicles after rehydration. These wet-dry cycles could also lead to the encapsulation of some of the solutes being trapped between the lamellas (Damer and Deamer 2015).
Figure 1.13. Vesicle formation in the prebiotic environment. (Source: Damer B, Deamer D (2015) Coupled Phases and Combinatorial Selection in Fluctuating Hydrothermal Pools: A Scenario to Guide Experimental Approaches to the Origin of Cellular Life. Life 5:872–887)
It has been established, that stable fatty-acid vesicles can grow and divide while maintaining the integrity of their contents (Fig. 1.14) (Zhu and Szostak 2009).
Since fatty acids are a likely molecular precursor to the modern lipids, there are numerous studies investigating how simple biomolecules can be transported inside of these compartments and how the membranes affect their behavior.
There is evidence that the presence of decanoic acid vesicles can invert the stereoselectivity and enhance the rate of peptide bond formation (Murillo- Sánchez et al. 2016). Another example is in the enhanced rate at which Ser-His catalyzes the production of a hydrophobic dipeptide in the presence of fatty acid bilayer. In this case, the dipeptide product binds to and promotes the growth of the vesicles, creating a simple form of mutualism in the system (Adamala and Szostak 2013a).
Figure 1.14. The growth and division of the multilamellar vesicles after addition of external micelles. The Epifluorescence micrographs show from left to right the evolution of oleic acid vesicles (left micrograph) after micelles addition.
(Source: Zhu TF, Szostak JW (2009) Coupled Growth and Division of Model Protocell Membranes. J Am Chem Soc 131:5705–5713)
The encapsulation inside of vesicles by amphiphilic membrane-like structures may be beneficial to the encapsulated system by offering protection from hydrolysis and by preventing loss of the system through diffusion, as has been demonstrated for example by the chemical replication of RNA inside fatty acid vesicles (Adamala and Szostak 2013b) and by protein translation within vesicles that contain the 80 different macromolecular species required for translation (Souza et al. 2009).
Therefore, the presence of simple fatty acids vesicles could be beneficial in many prebiotic processes. By providing semipermeable membranes that are able to grow and divide while retaining the internalized components, vesicles can protect their interior from an unfavorable external environment and transport encapsulated systems into other environments where they could evolve and increase in complexity. Furthermore, the hydrophobic bilayer provides an environment for the reactions that could not be performed in purely aqueous conditions. Taken all together, fatty acid vesicles could have played a significant role in the origins of life.
2. Carbonyl sulfide as a prebiotic activation agent for stereo- and sequence -selective, amyloid-templated peptide elongation
This chapter has been published as “Carbonyl sulfide as a prebiotic activation agent for stereo- and sequence-selective, amyloid-templated peptide elongation”
in Origins of Life and Evolution of Biospheres (Bomba et al. 2019). The authors of the publication are Radoslaw Bomba, Saroj K. Rout, Matthias Bütikofer, Witek Kwiatkowski, Roland Riek and Jason Greenwald. The co-authors agreed to the use of this publication in this thesis. Necessary adaptations have been made in order to integrate the publication into the format of this thesis.
Author contributions: RB, SR, WK, RR and JG conceived and designed the experiments. RB, MB and SR carried out the experiments. All authors contributed to the writing of the manuscript.
2.1. Abstract
Prebiotic chemical replication is a commonly assumed precursor to and prerequisite for life and as such is the one of the goals of our research. We have previously reported on the role that short peptide amyloids could have played in a template-based chemical elongation. Here we take a step closer to the goal by reproducing amyloid-templated peptide elongation with carbonyl sulfide (COS) in place of the less-prebiotically relevant carbonyldiimidazole (CDI) used in the earlier study. Our investigation shows that the sequence-selectivity and stereoselectivity of the amyloid-templated reaction is similar for both activation chemistries. Notably, the amyloid protects the peptides from some of the side- reactions that take place with the COS-activation.
2.2. Introduction
In the last years protein amyloids have been the subject of numerous lines of research that have uncovered novel biological functions in all forms of life (Chiti and Dobson 2006; Greenwald and Riek 2010). The formation of amyloid fibrils has also long been associated with many diseases including Alzheimer’s and Parkinson’s diseases (Chiti and Dobson 2006; Greenwald and Riek 2010).
Amyloids could also have played an important role in the origin of life, as we and others have recently hypothesized (Carny and Gazit 2005; Dale 2006;
Maury 2009; Greenwald and Riek 2012). They can be stable in harsh conditions and have inherent templating and stereoselective properties (Meersman and Dobson 2006; Childers et al. 2009; Omosun et al. 2017). As shown already in many studies, amyloids formed by short peptides can have catalytic activities (Reches and Gazit 2003; Mehta et al. 2008; Lara et al. 2013; Rufo et al. 2014;
Zhang et al. 2014; Friedmann et al. 2015; Makhlynets et al. 2016) and are able to form highly organized tube-like structures both in isolation (Omosun et al.
2017) and cooperatively upon interaction with other prebiotically relevant entities like fatty acids (Reches and Gazit 2003; Mehta et al. 2008; Lara et al.
2013; Sánchez-Ferrer et al. 2018; Bomba et al. 2018). Our recent findings, showing that the amyloid structure can support the template-directed synthesis of peptides (Rout et al. 2018), is just one of the latest results that strengthens the hypothesis that amyloids were involved in the origin of life. It should be noted that unraveling the mystery of the origin of life is not a question of a few key experiments but rather the synthesis of many smaller steps into hypotheses that can be further refined and tested.
We previously demonstrated that by forming an ordered aggregate, a complementary pair of amyloidogenic peptides can provide a structural scaffold that enhances peptide elongation via sequence-selective, regio-selective and stereoselective addition of activated amino acids (Rout et al. 2018). These results were obtained using amino acids that had been activated by CDI. CDI provides good yields of peptide addition products with few side reactions and was therefore chosen for the initial experiments. However, in pursuit of our goal of
a prebiotic chemical replication, we have now chosen to use carbonyl sulfide (COS), a volcanic gas that has been shown to act as a condensation agent for amino acids (Leman et al. 2004), in the amyloid templating system. The proposed mechanism of amino acid activation via COS has several intermediates, but the most reactive species, the N-carboxy anhydride, is likely to be the same as for the activation by CDI (Ehler and Orgel 1976; Leman et al.
2004). Indeed, our new results indicate that the amyloid template-assisted peptide syntheses with either COS- or CDI-activated amino acids are very similar.
2.3. Results and discussion
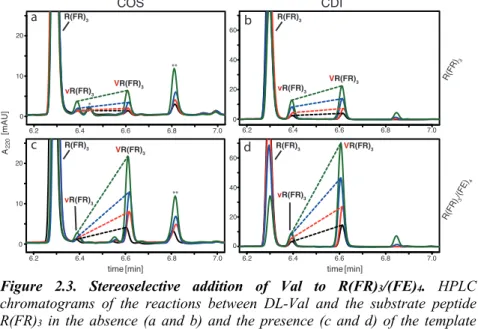
2.3.1. Stereoselective addition of amino acids to R(FR)
3templated by (FE)
4In this study we investigate a system of complementary substrate-template amyloid peptides that was previously shown to have a templating capability (Rout et al. 2018). The substrate peptide RFRFRFR-NH2, abbreviated R(FR)3, is the charge complement of the template peptide Ac-FEFEFEFE-NH2, abbreviated (FE)4. Both peptides have C-terminal amides and the template peptide is acetylated on its N-terminus. Since the substrate has one Phe residue less, the amyloid formed by these two peptides is selective for additions at the N-terminus of the substrate peptide. Both peptides were shown to be soluble above 1 mM at neutral pH, while the mixture of 100 µM of each peptide in 20 mM phosphate buffer at pH 7.4 yields an insoluble amyloid (Rout et al. 2018).
In order to assay the stereoselectivity of the R(FR)3/(FE)4 amyloid, racemic Phe was activated with COS and added at concentrations from 50 to 500 µM (25- 250 µM of each enantiomer) to either the amyloid aggregate R(FR)3/(FE)4 or the soluble substrate R(FR)3 alone. All reactions contained 1 mM potassium hexacyanoferrate as an oxidizing agent that increases the rate of the
condensation. After 30 min, the reactions were solubilized with guanidine hydrochloride (GuHCl) and analyzed by reversed phase HPLC. In order to properly compare the two activation chemistries, we also performed all of the reactions with the CDI activation as previously reported (Rout et al. 2018). As depicted in the chromatograms in Fig. 2.1 and tabulated in Table 2.1, the enhanced stereoselectivity and yield of addition of L-Phe to the substrate is independent of the type of activation (COS or CDI). Also, both chemistries give a significantly enhanced yield of the product (FR)4 formed in the amyloid- templated reactions compared to reactions with soluble substrate.
Table 2.1. Stereoselectivity and yield of amino acid addition to R(FR)3 and R(FR)3/(FE)4.
Amino
acid Concentration
[µM] (FE)4 COS activation CDI activation
dra(L/D) yieldb [%] dr(L/D) yield[%]
DL-Phe
50 - 1.5 1.9 1.6 6.9
+ 2.3 3.8 3.2 19
100 - 1.5 3.8 1.7 13
+ 2.5 8.7 3.8 34
200 - 1.8 3.6 1.9 20
+ 3.8 19 5.2 52
500 - 1.8 9.4 2.4 28
+ 6.2 31 8.1 53
DL-Val
50 - 1.6 1.3 1.6 4.6
+ 4.6 3.2 4.8 13
100 - 1.7 2.1 1.8 8.0
+ 6.3 6.2 4.8 24
200 - 1.9 3.3 1.8 16
+ 6.9 10 5.8 41
500 - 2.0 6.5 2.0 26
+ 7.0 17 8.1 60
DL-Leu
50 - 2.0 3.5 1.6 6.4
+ 2.3 6.4 2.4 16
100 - 1.8 5.3 1.5 11
+ 2.5 12 2.8 26
200 - 1.7 7.9 1.6 18
+ 3.0 20 3.3 36
500 - 1.6 12 1.6 24
+ 5.1 31 4.6 49
DL-Trp
50 - 1.2 0.9 2.9 8.1
+ 2.4 3.7 3.3 27
100 - 1.2 1.5 3.3 12
+ 2.8 5.6 4.4 40
200 - 1.2 2.8 4.3 19
+ 3.4 12 6.6 47
500 - 1.7 4.9 6.6 23
+ 3.1 10 9.2 30
DL-Tyr
50 - --c <1 1.5 2.2
+ -- 3.3 2.0 5.6
100 - -- <1 1.5 4.0
+ -- 3.8 2.6 11
200 - -- <1 1.4 6.8
+ -- 3.3 3.0 15
500 - -- <1 1.5 11
+ -- 2.8 4.2 23
aDiastereomeric ratio dr is the ratio of single L- versus D- addition products.
b Yield was calculated as the amount of single addition product relative to the amount of R(FR)3
used in the reaction.
cThe low yield of Tyr addition products relative to the side products of COS-activation made an analysis of the dr not possible.
While the stereoselectivity of the templated reactions was similar to the previous results with CDI activation, we were surprised to see that the stereoselectivity of the non-templated soluble peptide was substantially higher than previously reported (Rout et al. 2018), although still significantly lower than in the templated addition reaction (Fig. 2.1). Upon further investigation we found that the reason for the apparent change in stereoselectivity in the non-templated reaction was a variable loss of the L-Phe addition products, attributed to poor product recovery. Compared to the D-Phe addition product fR(FR)3, the L-Phe addition product (FR)4 has higher binding affinity to the plastic walls of the reaction tubes. This causes a selective partial loss of one of the diastereomeric products which results in an apparent lower diastereomeric excess of the L-Phe addition product. By performing all subsequent reactions in Protein LoBind Tubes (Eppendorf) we were able to get a consistently higher recovery of (FR)4
from all of the reactions, thereby minimizing the effect from the loss of the peptide to the tube walls. The total peptide recovery of each reaction was routinely monitored to control for the potential artifacts of poor product recovery. The total peptide recovery is the amount of substrate peptide and its addition products that were detected by HPLC in the reaction compared to the amount of substrate detected in a control reaction without activation. The main result is the same when using the LoBind tubes: the addition of racemic Phe to the R(FR)3/(FE)4 amyloid is significantly more stereoselective for L-Phe addition than the non-templated reaction with soluble peptide.
The enhancement in stereoselectivity in the amyloid-templated reactions gives an approximately 1.5 to 3x increase in the diastereomeric ratio (dr) of L to D- addition products compared to non-templated reaction, regardless of the activation chemistry (CDI or COS) (Fig. 2.1). As previously observed for CDI activated amino acids, the dr is dependent on the Phe concentration in the reaction, indicating that the reaction with COS follows the same or very similar mechanism as for CDI. We previously reported that the templated reaction is not simply first order with respect to L-Phe, while the non-templated and D-Phe addition reactions are first order with respect to Phe. Based on a kinetic analysis, we have proposed that for the L-Phe addition to the amyloid, the stereoselection
involves an additional binding event via a reaction intermediate (Rout et al.
2018).
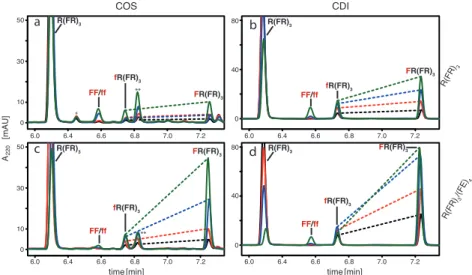
Figure 2.1. Stereoselective addition of phenylalanine to the R(FR)3/(FE)4
amyloid. HPLC chromatograms of the reactions between DL-Phe and the substrate peptide R(FR)3 in the absence (a and b) and the presence (c and d) of the template peptide (FE)4. The Phe was activated with either COS (a and c) or CDI (b and d). The color of the traces indicates four different concentrations of activated DL-Phe: 50 µM (black); 100 µM (red); 200 µM (blue) and 500 µM (green). The identity of the peaks that have been confirmed by standards and or mass spectrometry are labelled using lower case letters to indicate D-amino acid addition products. The dashed lines connect the peaks of fR(FR)3 and (FR)4
from the same chromatogram, highlighting the enhanced stereoselectivity of the amyloid-templated addition reactions. Peaks indicated by “*” come from the side products formed in COS reactions (described in the main text).
The absolute yield of the reactions with COS are lower than those with CDI (Table 2.1) which is to be expected considering the generally lower yields of peptide bonds via COS versus CDI activation. In fact, the templated reaction with CDI-activated Phe is so efficient that compared to the reaction with 200 µM Phe, the 500 µM reaction has a decrease in yield of the single addition
time [min]
A220[mAU]
time [min]
50
30
10
0
50
30
10
0
80
40
0
80
40
0
6.0 6.4 6.6 6.8 7.0 7.2
a b
c
COS CDI
R(FR) /(FE)3
4
R(FR)
3
R(FR)3
fR(FR)3 FF/ff
FR(FR)3
6.0 6.4 6.6 6.8 7.0 7.2
d
6.0 6.4 6.6 6.8 7.0 7.2
6.0 6.4 6.6 6.8 7.0 7.2
R(FR)3
fR(FR)3 FF/ff
FR(FR)3 R(FR)3
fR(FR)3
FF/ff FR(FR)3
R(FR)3
fR(FR)3 FF/ff
FR(FR)3
*
**
**